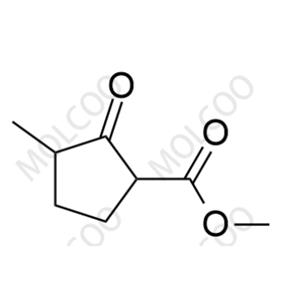
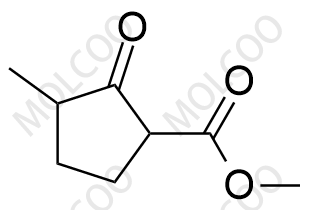
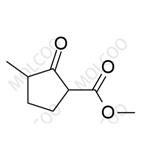
Product Details
| Product Name: Loxoprofen Impurity 80 | CAS No.: 57964-61-1 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
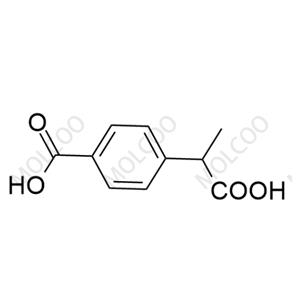
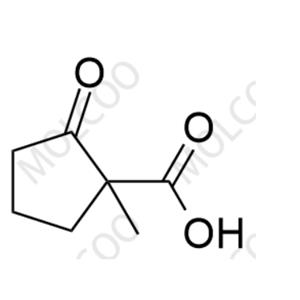
Loxoprofen, a prodrug-type nonsteroidal anti-inflammatory drug (NSAID), exerts analgesic, anti-inflammatory, and antipyretic effects via metabolic conversion to its active trans-OH form. In pharmaceutical R&D and quality control, impurity reference standards are critical for ensuring drug safety and efficacy. This documentation provides high-purity Loxoprofen impurity reference standards, covering major degradation products and process-related impurities (e.g., Impurity A, B, C), suitable for forced degradation studies, analytical method validation, and ICH Q3A/Q3B compliance.
Technical Specifications
| Parameter | Details |
|---|---|
| Purity | ≥98.0% (HPLC area normalization), single impurity ≤0.1% |
| Structural Confirmation | ¹H-NMR, ¹³C-NMR, MS, and HPLC spectra provided for structural elucidation |
| Packaging | 10mg/vial (customizable to 25mg, 50mg) |
| Stability | Inert gas packaging, stored at -20°C (dark), shelf life ≥3 years |
| Compliance | Aligned with ChP, USP, EP pharmacopoeias; includes CoA and DSC support |
Applications
Forced Degradation Studies: Simulate degradation under light, heat, and acid/base conditions to establish impurity profiles.
Method Validation: Support HPLC/UPLC method development for specificity, linearity, and LOQ/LOD studies.
Genotoxic Impurity Screening: Assess mutagenic potential per ICH M7 guidelines.
Drug Metabolism Research: Track metabolic pathways of Loxoprofen in vivo.
Representative Impurities
| Impurity Code | Chemical Name | CAS No. | Molecular Formula | Molecular Weight | Key Properties |
|---|---|---|---|---|---|
| Impurity A | Loxoprofen Acid (Active Metabolite) | 68767-14-6 | C₁₅H₁₈O₃ | 246.30 | Trans-OH configuration, core anti-inflammatory structure |
| Impurity B | Loxoprofen Ethyl Ester (Process Impurity) | 81623-55-0 | C₁₇H₂₂O₄ | 290.35 | Prodrug ester, less stable than parent drug |
| Impurity C | Loxoprofen Epoxide (Degradation Impurity) | 112853-53-5 | C₁₅H₁₆O₃ | 244.28 | Potential genotoxic impurity, strict control required |
| Impurity D | Loxoprofen Amide (Synthetic Intermediate) | 157293-95-3 | C₁₄H₁₇NO₂ | 231.29 | Used for synthetic route optimization |
Quality Assurance
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-17 | |
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2021-06-28 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-10 |









 China
China