
Product Details
| Product Name: Mirabegron Impurity 33 | CAS No.: 3017159-61-1 |
| Min. Order: 1mg | Purity: >95% HPLC |
| Supply Ability: 100000 | Release date: 2025/07/31 |
| Molecular Formula:: C21H23N5O3S | Molecular Weight:: 425.5 |
| Appearance: Yellow soild | Storage: 2-8°C Refrigerator |

| Product Catalog: | M005033 |
| CAS No.: | 3017159-61-1 |
| Product Name: | Mirabegron Impurity 33 |
| Purity: | >95% HPLC |
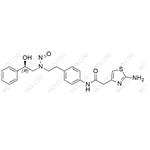
| Synonyms: | (R)-2-(2-aminothiazol-4-yl)-N-(4-(2-((2-hydroxy-2-phenylethyl)(nitroso)amino)ethyl)phenyl)acetamide |
| Molecular Formula: | C21H23N5O3S |
| Mol. Weight: | 425.5 |
| Appearance: | Yellow soild |
| Storage: | 2-8°C Refrigerator |
| Contact: | WhatsAPP: +86 17320513646 E-mail: anna@molcoo.com |
| Note: | We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS. This product is intended for laboratory use only! |
| Background: | Mirabegron Impurity 33 is an impurity associated with mirabegron, a medication used to treat overactive bladder. (R)-2-(2-aminothiazol-4-yl)-N-(4-(2-((2-hydroxy-2-phenylethyl)(nitroso)amino)ethyl)phenyl)acetamide is a complex organic compound with a multi - functional structure. Its structure contains thiazole, phenyl, and nitroso groups, among others.It may form during the synthesis process, storage, or degradation of mirabegron. Scientists conduct in - depth research to detect, analyze, and quantify this impurity. By controlling its levels, pharmaceutical companies can guarantee that mirabegron - based drugs meet strict regulatory standards, protecting patients from potential adverse effects caused by impurities. |
Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 | |
| $0.00/10g |
HangZhou RunYan Pharma Technology Co.,LTD.
|
2024-11-20 | ||
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-10-11 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-05-15 |
INQUIRY







 China
China