
Product Details
| Product Name: Montelukast Impurity 23 | CAS No.: 162515-66-4 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
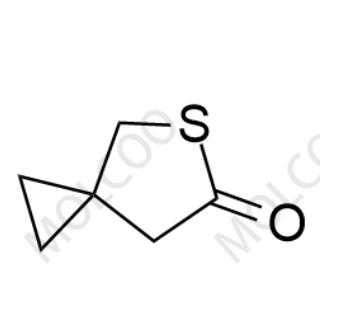
| Molecular formula: C6H8OS |
Montelukast Impurity 23
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: M012023
English Name: Montelukast Impurity 23
English Alias: 5-thiaspiro[2.4]heptan-6-one
CAS Number: 162515-66-4
Molecular Formula: C₆H₈OS
Molecular Weight: 128.19
Advantages
Well-defined and distinct structure: Contains a thiaspiro ring (5-thiaspiro[2.4]heptane) and ketone group, differing from montelukast by the absence of benzene rings and long side chains. The steric hindrance of the spiro structure results in significant differences in polarity and retention behavior from montelukast, enabling accurate identification via GC and HPLC as a specific marker for impurity detection;
High stability and traceability: The cyclic structure of the thiaspiro ring and the conjugation of the ketone group ensure high stability under non-alkaline conditions. As an intermediate derivative in the construction of the thiaspiro ring during montelukast synthesis, it directly reflects the reaction efficiency of the spiro ring formation step, improving the accuracy of process tracing;
High detection sensitivity: The electronegativity of the sulfur atom and the strong polarity of the ketone group produce characteristic responses in mass spectrometry (m/z 129 [M+H]⁺), combined with the absorption of the ketone group in the ultraviolet region (210-230nm), enabling trace analysis (ppm level) via GC-MS or HPLC-UV, compatible with impurity detection systems of leukotriene receptor antagonists containing thiazoles.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Montelukast Impurity 23 in montelukast API production, ensuring that residual intermediate impurities in the thiaspiro ring synthesis process meet quality standards;
Synthesis process optimization: By monitoring the content of this impurity, optimize the cyclization reaction conditions of the thiaspiro ring (such as catalyst type, reaction temperature) to improve the formation efficiency of the target spiro structure and reduce the generation of by-products;
Intermediate purity evaluation: Used to evaluate the purity of key thiaspiro intermediates in montelukast synthesis, provide data support for the specificity of subsequent benzene ring coupling reactions, and ensure the quality of the final product.
Background Description
Research Status
Detection method optimization: Using gas chromatography-mass spectrometry (GC-MS) technology, optimizing the chromatographic column selection (such as weakly polar capillary columns) by utilizing the volatility of the thiaspiro ring, achieving baseline separation between the impurity and montelukast intermediates, with a detection limit as low as 0.5 ppm;
Cyclization mechanism analysis: Studying the formation kinetics of the impurity by simulating different sulfur sources (such as thiols) and cyclization reagent conditions to clarify the key influencing factors of thiaspiro ring formation in montelukast;
Process scale-up verification: Monitoring the content of this impurity in large-scale production to optimize the reaction parameters of spiro ring synthesis (such as reaction time, solvent polarity) to ensure the stability of impurity content between batches;
Improvement of impurity profile: Incorporate this impurity into the impurity profile research of montelukast to provide data support for quality research in drug registration applications and meet regulatory requirements for impurity control
Product Information
Product Number: M012023
English Name: Montelukast Impurity 23
English Alias: 5-thiaspiro[2.4]heptan-6-one
CAS Number: 162515-66-4
Molecular Formula: C₆H₈OS
Molecular Weight: 128.19
Advantages
Well-defined and distinct structure: Contains a thiaspiro ring (5-thiaspiro[2.4]heptane) and ketone group, differing from montelukast by the absence of benzene rings and long side chains. The steric hindrance of the spiro structure results in significant differences in polarity and retention behavior from montelukast, enabling accurate identification via GC and HPLC as a specific marker for impurity detection;
High stability and traceability: The cyclic structure of the thiaspiro ring and the conjugation of the ketone group ensure high stability under non-alkaline conditions. As an intermediate derivative in the construction of the thiaspiro ring during montelukast synthesis, it directly reflects the reaction efficiency of the spiro ring formation step, improving the accuracy of process tracing;
High detection sensitivity: The electronegativity of the sulfur atom and the strong polarity of the ketone group produce characteristic responses in mass spectrometry (m/z 129 [M+H]⁺), combined with the absorption of the ketone group in the ultraviolet region (210-230nm), enabling trace analysis (ppm level) via GC-MS or HPLC-UV, compatible with impurity detection systems of leukotriene receptor antagonists containing thiazoles.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Montelukast Impurity 23 in montelukast API production, ensuring that residual intermediate impurities in the thiaspiro ring synthesis process meet quality standards;
Synthesis process optimization: By monitoring the content of this impurity, optimize the cyclization reaction conditions of the thiaspiro ring (such as catalyst type, reaction temperature) to improve the formation efficiency of the target spiro structure and reduce the generation of by-products;
Intermediate purity evaluation: Used to evaluate the purity of key thiaspiro intermediates in montelukast synthesis, provide data support for the specificity of subsequent benzene ring coupling reactions, and ensure the quality of the final product.
Background Description
Research Status
Detection method optimization: Using gas chromatography-mass spectrometry (GC-MS) technology, optimizing the chromatographic column selection (such as weakly polar capillary columns) by utilizing the volatility of the thiaspiro ring, achieving baseline separation between the impurity and montelukast intermediates, with a detection limit as low as 0.5 ppm;
Cyclization mechanism analysis: Studying the formation kinetics of the impurity by simulating different sulfur sources (such as thiols) and cyclization reagent conditions to clarify the key influencing factors of thiaspiro ring formation in montelukast;
Process scale-up verification: Monitoring the content of this impurity in large-scale production to optimize the reaction parameters of spiro ring synthesis (such as reaction time, solvent polarity) to ensure the stability of impurity content between batches;
Improvement of impurity profile: Incorporate this impurity into the impurity profile research of montelukast to provide data support for quality research in drug registration applications and meet regulatory requirements for impurity control
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-20 | |
| $0.00/1kg |
VIP2Y
|
Shaanxi Xianhe Biotech Co., Ltd
|
2025-05-09 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 |










 China
China