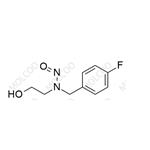
Product Number: N031279
English Name: N-nitroso-2-(4-fluorobenzylamino)ethanol
English Alias: N-(4-fluorobenzyl)-N-(2-hydroxyethyl)nitrous amide
CAS Number: None
Molecular Formula: C9H11FN2O2
Molecular Weight: 198.19
Advantages: N-nitroso-2-(4-fluorobenzylamino)ethanol achieves high purity and excellent batch stability through optimized synthesis routes and strict separation and purification processes. The product undergoes structural confirmation and content determination by various precise analytical methods, such as nuclear magnetic resonance and high-resolution mass spectrometry, ensuring accurate chemical structure and stable, uniform composition. It can provide a reliable reference substance for drug research, development, and quality control, effectively improving the accuracy and repeatability of experimental data.
Applications: It is mainly applied to the fields of impurity research, quality analysis, and control of related drugs. During the drug research and development process, it can be used to determine the content of this impurity in drugs and evaluate its potential impact on drug safety and effectiveness. In the quality control of pharmaceutical production, as a key reference substance, it can accurately detect the presence of impurities in products, helping enterprises strictly control drug quality and ensure compliance with relevant domestic and international regulations and quality standards. It also has important reference value in drug metabolism research and safety evaluation.
Background Description: With the increasingly stringent requirements for drug quality in the pharmaceutical industry, the research on drug impurities has become an important part of ensuring drug safety and efficacy. The presence of impurities may affect drug stability, efficacy, and even pose safety risks. N-nitroso-2-(4-fluorobenzylamino)ethanol, as a potential drug impurity, research on its properties, sources, and impacts helps to improve the drug quality control system, ensure patient medication safety, and enhance the overall quality of drugs.
Research Status: Currently, research on N-nitroso-2-(4-fluorobenzylamino)ethanol is gradually deepening. Researchers are committed to developing more sensitive and efficient detection methods, such as ultra-high performance liquid chromatography - tandem mass spectrometry (UPLC - MS/MS) and high-resolution gas chromatography - mass spectrometry (HRGC - MS), to achieve trace detection and precise quantification of this impurity. Meanwhile, studies on its generation mechanism during drug synthesis, its interaction with drug active ingredients, as well as its impact on drug stability and efficacy, are also actively carried out, aiming to provide more comprehensive theoretical support and technical guidance for drug research, development, production, and quality control.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!











 China
China