
Product Details
| Product Name: N-Nitroso Isoproterenol Impurity 1 | Min. Order: 10mg |
| Purity: 99%+ HPLC | Supply Ability: 1000 |
| Release date: 2025/07/31 | |
| Molecular formula: C11H16N2O4 |
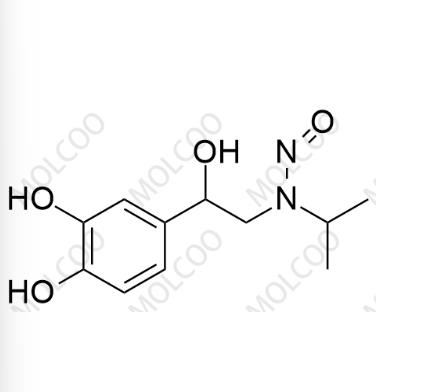
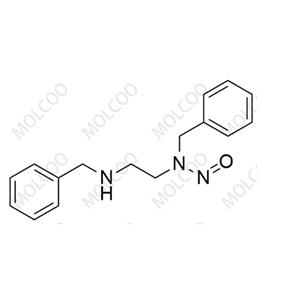
N-Nitroso Isoproterenol Impurity 1
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: I035008
English Name: N-Nitroso Isoproterenol Impurity 1
English Alias: N-(2-(3,4-dihydroxyphenyl)-2-hydroxyethyl)-N-isopropylnitrous amide
CAS Number: None
Molecular Formula: C₁₁H₁₆N₂O₄
Molecular Weight: 240.26
Advantages
With a well-defined structure and good stability, it can be precisely used to analyze the by-product formation mechanism of nitrosation reactions during isoproterenol synthesis or storage, optimizing processes to control the generation of nitroso impurities.
Containing multiple functional groups such as nitroso, phenolic hydroxyl, and alcoholic hydroxyl groups, it can provide a standard substance for the detection of nitroso impurities in drugs, significantly improving the quantitative accuracy of detection methods such as HPLC and LC-MS.
It helps to study the impact of nitroso and polyhydroxy structures on drug stability and toxicological properties, providing a scientific basis for impurity control strategies and ensuring drug safety.
Applications
Drug Development: During the research and development of isoproterenol and its formulations, it is used as an impurity reference standard to identify and quantitatively analyze N-Nitroso Isoproterenol Impurity 1, accurately evaluating the purity of APIs and formulations.
Quality Control: As a standard substance, it is used to verify the sensitivity and specificity of detection methods such as HPLC and LC-MS, ensuring that the content of this impurity during production meets pharmacopoeia and relevant regulatory requirements.
Toxicological Research: Assisting in evaluating the potential genotoxicity of nitroso impurities, providing data support for drug safety evaluation through in vitro cytotoxicity experiments and animal models.
Background Description
Research Status
Detection Method Optimization: Using advanced technologies such as ultra-high-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS) to develop highly sensitive and selective detection methods for trace detection of this impurity.
Synthesis Process Improvement: Deeply studying the formation pathway of this impurity, and developing synthesis processes that reduce impurity generation by optimizing reaction conditions (such as reaction temperature, time, solvent selection) and raw material ratios.
Toxicological Evaluation: Evaluating the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models, providing data support for scientifically formulating reasonable impurity limit standards.
Stability Studies: Systematically studying the stability of this impurity under the influence of different environmental factors (light, high temperature, high humidity), analyzing its impact on the quality of isoproterenol formulations, and further improving the drug quality control system.
Product Information
Product Number: I035008
English Name: N-Nitroso Isoproterenol Impurity 1
English Alias: N-(2-(3,4-dihydroxyphenyl)-2-hydroxyethyl)-N-isopropylnitrous amide
CAS Number: None
Molecular Formula: C₁₁H₁₆N₂O₄
Molecular Weight: 240.26
Advantages
With a well-defined structure and good stability, it can be precisely used to analyze the by-product formation mechanism of nitrosation reactions during isoproterenol synthesis or storage, optimizing processes to control the generation of nitroso impurities.
Containing multiple functional groups such as nitroso, phenolic hydroxyl, and alcoholic hydroxyl groups, it can provide a standard substance for the detection of nitroso impurities in drugs, significantly improving the quantitative accuracy of detection methods such as HPLC and LC-MS.
It helps to study the impact of nitroso and polyhydroxy structures on drug stability and toxicological properties, providing a scientific basis for impurity control strategies and ensuring drug safety.
Applications
Drug Development: During the research and development of isoproterenol and its formulations, it is used as an impurity reference standard to identify and quantitatively analyze N-Nitroso Isoproterenol Impurity 1, accurately evaluating the purity of APIs and formulations.
Quality Control: As a standard substance, it is used to verify the sensitivity and specificity of detection methods such as HPLC and LC-MS, ensuring that the content of this impurity during production meets pharmacopoeia and relevant regulatory requirements.
Toxicological Research: Assisting in evaluating the potential genotoxicity of nitroso impurities, providing data support for drug safety evaluation through in vitro cytotoxicity experiments and animal models.
Background Description
Research Status
Detection Method Optimization: Using advanced technologies such as ultra-high-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS) to develop highly sensitive and selective detection methods for trace detection of this impurity.
Synthesis Process Improvement: Deeply studying the formation pathway of this impurity, and developing synthesis processes that reduce impurity generation by optimizing reaction conditions (such as reaction temperature, time, solvent selection) and raw material ratios.
Toxicological Evaluation: Evaluating the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models, providing data support for scientifically formulating reasonable impurity limit standards.
Stability Studies: Systematically studying the stability of this impurity under the influence of different environmental factors (light, high temperature, high humidity), analyzing its impact on the quality of isoproterenol formulations, and further improving the drug quality control system.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-30 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $0.00/10KG |
VIP2Y
|
Shandong Hanjiang Chemical Co., Ltd
|
2025-10-09 |








 China
China