Product Details
| Product Name: Oseltamivir EP Impurity 148 | CAS No.: 2884522-60-3 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C14H23ClO4 |
Oseltamivir EP Impurity 148;2884522-60-3

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
- Product Information
Product Code: O011148
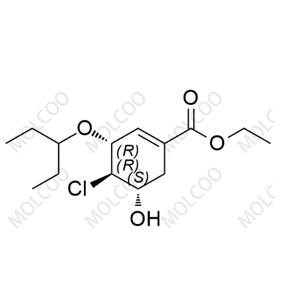
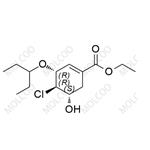
English Name: Oseltamivir EP Impurity 148
English Alias: (3R,4R,5S)-ethyl 4-chloro-5-hydroxy-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate
CAS Number: 2884522-60-3
Molecular Formula: C14H23ClO4
Molecular Weight: 290.78
Advantages
As a specific impurity reference standard for oseltamivir, it has a clear chemical structure and a known CAS number, facilitating accurate qualitative and quantitative analysis.
With definite molecular weight and formula, it helps laboratories identify and verify the impurity through methods such as mass spectrometry and chromatography.
Complies with the relevant impurity research standards of the European Pharmacopoeia (EP), suitable for standardized pharmaceutical quality control processes.
Applications
Mainly used in the quality control of oseltamivir active pharmaceutical ingredients (APIs) and preparations. As an impurity reference standard, it assists in detecting this specific impurity that may be generated during production or storage.
Can be applied in impurity profile analysis during drug development, helping researchers understand the degradation pathways or synthetic by-products of oseltamivir.
Suitable for the development and validation of analytical methods in pharmaceutical laboratories to ensure the sensitivity and accuracy of the detection method for this impurity.
Background Description
Research Status
Product Code: O011148
English Name: Oseltamivir EP Impurity 148
English Alias: (3R,4R,5S)-ethyl 4-chloro-5-hydroxy-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate
CAS Number: 2884522-60-3
Molecular Formula: C14H23ClO4
Molecular Weight: 290.78
Advantages
As a specific impurity reference standard for oseltamivir, it has a clear chemical structure and a known CAS number, facilitating accurate qualitative and quantitative analysis.
With definite molecular weight and formula, it helps laboratories identify and verify the impurity through methods such as mass spectrometry and chromatography.
Complies with the relevant impurity research standards of the European Pharmacopoeia (EP), suitable for standardized pharmaceutical quality control processes.
Applications
Mainly used in the quality control of oseltamivir active pharmaceutical ingredients (APIs) and preparations. As an impurity reference standard, it assists in detecting this specific impurity that may be generated during production or storage.
Can be applied in impurity profile analysis during drug development, helping researchers understand the degradation pathways or synthetic by-products of oseltamivir.
Suitable for the development and validation of analytical methods in pharmaceutical laboratories to ensure the sensitivity and accuracy of the detection method for this impurity.
Background Description
Research Status
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-06-13 | |
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2020-05-31 | |
| $0.00/25KG |
VIP6Y
|
Sichuan Zhuoyu Yantang Technology Co., Ltd.
|
2025-08-08 |








 China
China