Ozanimod Impurity2251699 - 84 - 8
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information
Product Number: O036044
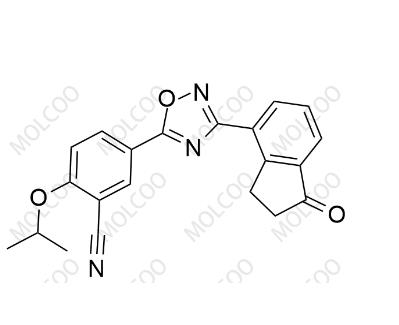
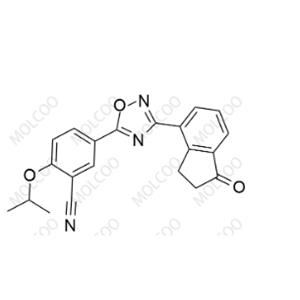
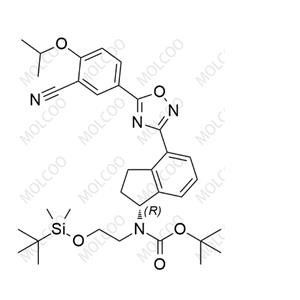
English Name: Ozanimod Impurity 44
English Alias: 2 - isopropoxy - 5-(3-(1 - oxo - 2,3 - dihydro - 1H - inden - 4 - yl)-1,2,4 - oxadiazol - 5 - yl)benzonitrile
CAS Number: 2251699 - 84 - 8
Molecular Formula: C21H17N3O3
Molecular Weight: 359.38
Advantages: As a reference standard for Ozanimod Impurity 44, it has an accurate and clear chemical structure, and has undergone strict purity testing and quality control, with good stability. It can maintain stable properties under different storage conditions and experimental environments. It can be used as a reliable reference substance to ensure the accuracy and repeatability of impurity detection results for Ozanimod-related products, helping pharmaceutical companies and research institutions to precisely control drug quality and providing strong support for quality assessment in the drug research and production process.
Applications: It is mainly used in the quality research, impurity analysis, and quality control of Ozanimod bulk drugs and formulations. It can be used to establish and validate impurity detection methods such as high-performance liquid chromatography (HPLC) and liquid chromatography - mass spectrometry (LC - MS). In the research and development process of Ozanimod, it is used to study the source and formation mechanism of impurities, and optimize the synthesis process to reduce impurity generation. In the production process, it is used to monitor the content of this impurity in products to ensure that drug quality meets relevant standards and regulatory requirements. It can also be used to evaluate the changes of impurities in Ozanimod drugs during storage and transportation, providing data support for the stability research of drugs.
Background Description: Ozanimod is a drug used for the treatment of immune-related diseases. In the process of its research and development and production, the presence of impurities may affect the safety, effectiveness, and stability of the drug. To ensure the safety of patients' medication and meet the requirements of drug regulation, strict research and control of impurities in Ozanimod are essential. As one of the impurities of Ozanimod, in-depth research on Ozanimod Impurity 44 helps to improve the quality standards of Ozanimod, enhance drug quality, and promote the drug research and development process.
Research Status: Currently, the research on Ozanimod Impurity 44 mainly focuses on the optimization and improvement of impurity analysis methods. By using more advanced detection technologies and instruments, the sensitivity and accuracy of detecting this impurity are improved to achieve precise determination of trace impurities. At the same time, researchers are actively exploring the source and change rules of this impurity during the synthesis and storage of Ozanimod, and reducing the generation and accumulation of impurities by improving process conditions and optimizing the storage environment. In addition, the research on the potential impact of this impurity on the performance and safety of Ozanimod drugs is also gradually underway, aiming to provide a more co
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!









 China
China