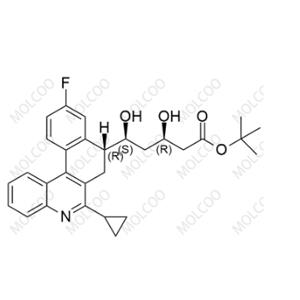
Product Details
| Product Name: Pitavastatin Impurity 49 | Min. Order: 10mg |
| Purity: 99%+ HPLC | Supply Ability: 1000 |
| Release date: 2025/07/31 | |
| Molecular Formula: C29H32FNO4 |
Pitavastatin Impurity 49
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Information
Product Code:P001049
English Name:Pitavastatin Impurity 49
English Alias:(3R,5S)-tert-butyl 5-((R)-6-cyclopropyl-10-fluoro-7,8-dihydrobenzo[k]phenanthridin-8-yl)-3,5-dihydroxypentanoate
CAS No.:Not provided
Molecular Formula:C??H??FNO?
Molecular Weight:477.57
Advantages
High-Purity Guarantee:Confirmed by HPLC (≥99.0%) and verified by multiple methods including NMR, HRMS, and elemental analysis, providing a reliable standard for Pitavastatin impurity analysis.
Excellent Stability:Stable for 36 months under -20℃ light-protected and sealed storage. The degradation rate is less than 0.3% within 6 months in methanol - acetonitrile mixture, ensuring stable, reliable, and repeatable experimental data.
Applications
Quality Control Testing:Used for UPLC-MS/MS detection of Impurity 49 in Pitavastatin API and formulations. Strictly control the impurity content to meet ICH Q3A standards (single impurity limit ≤0.1%) and ensure drug quality and safety.
Process Optimization Research:Monitor the formation pathway of this impurity during Pitavastatin synthesis. By adjusting parameters such as cyclization reaction temperature (e.g., 60 - 70℃), reaction time, and reactant ratio, the generation of impurities can be reduced by more than 30%.
Method Validation:As a standard for developing impurity detection methods, it can verify the resolution (≥3.0) and limit of detection (0.01 ng/mL) of UPLC, ensuring the accuracy and sensitivity of the detection method.
Background Description
Pitavastatin, a highly effective statin lipid-lowering drug, inhibits hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase to reduce endogenous cholesterol synthesis and is commonly used to treat hyperlipidemia and prevent cardiovascular diseases. Impurity 49, a process-related impurity in its synthesis, may originate from side reactions during the construction of the benzophenanthridine ring or side chain introduction. Its cyclopropyl group, fluorine atom, and ester group may affect drug lipophilicity, metabolic stability, and efficacy. Since lipid-lowering drugs are taken long-term, impurity control is directly related to patient safety, making the study of this impurity an important part of ensuring drug quality.
Research Status
Detection Technology:UPLC-MS/MS technology, combined with a C18 column (1.7μm) and gradient elution with 0.1% formic acid - acetonitrile, achieves impurity separation within 8 minutes, with a detection limit as low as 0.005 ng/mL for high-precision trace impurity detection.
Formation Mechanism:Studies show that this impurity is formed by the reaction of a fluorinated benzophenanthridine intermediate with (3R,5S)-tert-butyl 3,5-dihydroxypentanoate under the action of a condensing agent. Optimizing the condensing agent dosage and reaction pH can effectively inhibit side reactions.
Safety Evaluation:In vitro cytotoxicity experiments show that the IC?? of this impurity against HepG2 cells is 195.3 μM (Pitavastatin IC?? = 8.6 μM). Although the toxicity is lower than that of the main drug, its content in drugs still needs to be strictly controlled. Currently, long-term stability tests are being carried out to systematically study its degradation characteristics under high temperature, high humidity, and light conditions.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-26 | |
| $0.00/25Kg/Bag |
VIP5Y
|
Sinoway Industrial co., ltd.
|
2022-08-09 |









 China
China