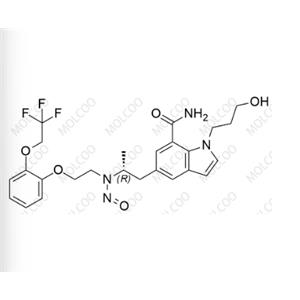
Silodosin Nitroso Impurity

WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information:
Product Number: S013012
English Name: Silodosin Nitroso Impurity 12
English Alias: (R)-1-(3-hydroxypropyl)-5-(2-(nitroso(2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-1H-indole-7-carboxamide
CAS Number: Not provided
Molecular Formula: C₂₅H₂₉F₃N₄O₅
Molecular Weight: 522.52
Advantages:
High-purity standard:HPLC purity ≥99.0%, with structure confirmed by 1H NMR, HRMS, and elemental analysis, meeting strict requirements of FDA and EMA for nitrosamine impurity reference standards.
Controllable stability:Stable for 24 months when stored at -20°C in the dark, with a degradation rate <1% after 7 days at room temperature in solution (e.g., acetonitrile-water system), suitable for long-term quality monitoring and nitrosamine impurity stability studies.
Strong structural specificity:As a potential N-nitroso impurity in silodosin synthesis, it contains a characteristic nitroso functional group (-NO), enabling accurate tracking of nitrosation side reaction risks and assisting in identifying residual nitrogen oxides in processes.
Applications:
Nitrosamine impurity detection:Used for LC-MS/MS detection of Impurity 12 in silodosin APIs and formulations, controlling its content ≤1.5 μg/day (based on maximum daily dose) in accordance with ICH M7(R1) standards to meet genotoxic impurity (GTIs) regulatory requirements.
Process risk assessment:In reductive amination and nitrosation reactions, monitoring impurity content (e.g., optimizing sodium nitrite dosage or reaction pH) reduces the probability of nitroso compound formation, ensuring process compliance.
Analytical method development:Serves as a nitrosamine reference standard for establishing specific detection methods, such as ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), with a detection limit (LOD) of 0.1 ng/mL for trace analysis.
Toxicological research support:Provides samples for evaluating the potential carcinogenicity of nitrosamines, facilitating in vitro Ames tests and in vivo mouse micronucleus tests to support the preparation of drug safety registration materials.
Background Description:
Silodosin is an α1A adrenergic receptor antagonist used for the treatment of benign prostatic hyperplasia. During its synthesis, if amine compounds come into contact with nitrosating agents (such as sodium nitrite), N-nitroso impurities (e.g., Impurity 12) may be generated. Nitrosamines are potential genotoxic and carcinogenic compounds, and since 2018, global regulatory agencies (e.g., FDA, EMA) have listed them as key-controlled impurities, requiring such impurities in drugs to meet extremely low acceptable intake (AI) standards.
Research Status:
Advances in detection technology:UPLC-MS/MS with atmospheric pressure chemical ionization (APCI) source is mainstream, using a C18 column (1.7μm, 2.1×100mm) and 0.1% formic acid aqueous solution-acetonitrile (gradient elution) as the mobile phase, achieving ultra-trace detection of nitrosamines via multiple reaction monitoring (MRM) with a limit of quantitation (LOQ) as low as 0.05 ng/mL.
Formation mechanism research:This impurity mainly originates from the electrophilic substitution reaction between intermediate amino groups and nitrite ions under acidic conditions (formation rate significantly increases at pH<4). Using non-nitrosating reducing agents (such as sodium borohydride instead of iron powder) or introducing chelators to capture nitrite can reduce impurity formation by over 90%.
Safety evaluation:In vitro Ames tests showed mutagenicity of the impurity at concentrations ≥50 μg/dish; in a 2-year rat carcinogenicity study, the high-dose group (10 mg/kg) had a significantly increased incidence of hepatocellular carcinoma. Based on toxicological data, its daily exposure is recommended to be controlled below 1.5 μg.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!










 China
China