
Product Details
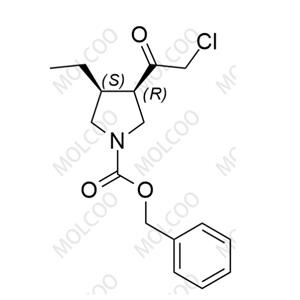
| Product Name: Upadacitinib Impurity 125 | CAS No.: 2304514-60-9 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/07/31 |

Upadacitinib Impurity Reference Standards —— Quality Assurance, Cornerstone of Scientific Research
In the meticulous world of drug research and production, Upadacitinib, as a new generation of selective JAK inhibitor, is leading a new chapter in the treatment of rheumatoid arthritis and certain dermatological conditions. However, the exceptional efficacy of any drug is built upon rigorous control of its impurities. Recognizing this, we have specially introduced Upadacitinib impurity reference standards, designed to assist researchers and quality control personnel in accurately identifying and quantifying impurity components in the drug.
Our Upadacitinib impurity reference standard series covers a variety of key impurities that may arise during the synthesis of raw materials and the preparation of finished products, including but not limited to isomers, oxidation products, and degradation products of Upadacitinib. Each impurity reference standard is meticulously prepared and undergoes strict quality testing to ensure its purity, structural stability, and batch-to-batch consistency meet the highest international standards.
By choosing our Upadacitinib impurity reference standards, you will receive:
Precise Identification: Enabling researchers to quickly pinpoint impurity types in the drug.
Quantitative Analysis: Providing reliable impurity content data for drug quality control.
Scientific Research Support: Facilitating new drug development and enhancing drug safety and efficacy.
Compliance Assurance: Meeting the requirements of domestic and international drug regulatory agencies to facilitate product launch.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/25kg |
VIP1Y
|
Shaanxi Dideu New Materials Co. Ltd
|
2025-06-19 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-01 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-03-29 |








 China
China