Product Details
| Product Name: Vismodegib Impurity 4 | CAS No.: 1370525-64-6 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C19H14Cl2N2O4S |
Vismodegib Impurity 4;1370525-64-6

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: V043004
English Name: Vismodegib Impurity 4
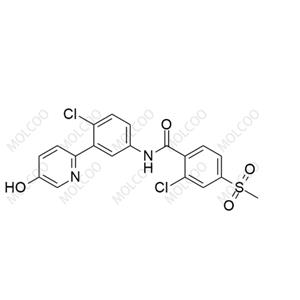
English Alias: 2-chloro-N-(4-chloro-3-(5-hydroxypyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide
CAS Number: 1370525-64-6
Molecular Formula: C₁₉H₁₄Cl₂N₂O₄S
Molecular Weight: 437.30
Advantages
Well-defined and highly characteristic structure: The unique structure with a hydroxyl group introduced at the 5-position of the pyridine ring is significantly different from the parent Vismodegib, containing functional groups such as chlorine atoms, hydroxyl groups, methylsulfonyl groups, and pyridine rings. It can be accurately identified by chromatographic and mass spectrometric techniques, providing a specific marker for impurity detection;
High stability and traceability: The hydroxylated pyridine ring structure is stable under conventional storage conditions and directly related to the metabolic pathway of Vismodegib. It can specifically reflect the degradation trend of the drug under enzyme catalysis or oxidation conditions, improving the accuracy of impurity source analysis;
Outstanding value as a detection standard: As a key impurity in the synthesis or metabolism of Vismodegib, its structural characteristics provide a standard for establishing exclusive detection methods, enabling effective differentiation between the parent drug and other hydroxylated by-products and improving quantitative precision.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Impurity 4 in Vismodegib APIs and formulations, ensuring product purity meets pharmacopoeia and regulatory requirements;
Stability studies: Monitoring the generation of this impurity under light, high temperature, and oxidation conditions to evaluate the storage stability of Vismodegib formulations and support the development of appropriate packaging and storage conditions;
Metabolic mechanism research: As a potential product of Vismodegib's in vivo pyridine ring hydroxylation metabolism, it is used to explore the drug's metabolic pathway in the liver and analyze the impact of hydroxylation on drug activity and toxicity.
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS technology to optimize separation conditions based on the polarity difference of pyridine ring hydroxyl groups, achieving trace detection of this impurity (detection limit up to ppb level);
Synthesis process improvement: Reducing the generation of hydroxylated by-products by optimizing the feeding ratio and reaction temperature of pyridine ring derivatives;
Toxicological evaluation: Comparing the inhibitory activity of this impurity and Vismodegib on the Hedgehog signaling pathway through in vitro cell experiments to assess its potential pharmacological interference or toxic effects;
Metabolic pathway analysis: Elucidating the in vivo generation rate and clearance pathway of this impurity through liver microsome experiments to provide a basis for drug safety evaluation
Product Information
Product Number: V043004
English Name: Vismodegib Impurity 4
English Alias: 2-chloro-N-(4-chloro-3-(5-hydroxypyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide
CAS Number: 1370525-64-6
Molecular Formula: C₁₉H₁₄Cl₂N₂O₄S
Molecular Weight: 437.30
Advantages
Well-defined and highly characteristic structure: The unique structure with a hydroxyl group introduced at the 5-position of the pyridine ring is significantly different from the parent Vismodegib, containing functional groups such as chlorine atoms, hydroxyl groups, methylsulfonyl groups, and pyridine rings. It can be accurately identified by chromatographic and mass spectrometric techniques, providing a specific marker for impurity detection;
High stability and traceability: The hydroxylated pyridine ring structure is stable under conventional storage conditions and directly related to the metabolic pathway of Vismodegib. It can specifically reflect the degradation trend of the drug under enzyme catalysis or oxidation conditions, improving the accuracy of impurity source analysis;
Outstanding value as a detection standard: As a key impurity in the synthesis or metabolism of Vismodegib, its structural characteristics provide a standard for establishing exclusive detection methods, enabling effective differentiation between the parent drug and other hydroxylated by-products and improving quantitative precision.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Impurity 4 in Vismodegib APIs and formulations, ensuring product purity meets pharmacopoeia and regulatory requirements;
Stability studies: Monitoring the generation of this impurity under light, high temperature, and oxidation conditions to evaluate the storage stability of Vismodegib formulations and support the development of appropriate packaging and storage conditions;
Metabolic mechanism research: As a potential product of Vismodegib's in vivo pyridine ring hydroxylation metabolism, it is used to explore the drug's metabolic pathway in the liver and analyze the impact of hydroxylation on drug activity and toxicity.
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS technology to optimize separation conditions based on the polarity difference of pyridine ring hydroxyl groups, achieving trace detection of this impurity (detection limit up to ppb level);
Synthesis process improvement: Reducing the generation of hydroxylated by-products by optimizing the feeding ratio and reaction temperature of pyridine ring derivatives;
Toxicological evaluation: Comparing the inhibitory activity of this impurity and Vismodegib on the Hedgehog signaling pathway through in vitro cell experiments to assess its potential pharmacological interference or toxic effects;
Metabolic pathway analysis: Elucidating the in vivo generation rate and clearance pathway of this impurity through liver microsome experiments to provide a basis for drug safety evaluation
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-07-24 | |
| $0.00/1kg |
VIP1Y
|
Jinan Ruitong Biotech Co., Ltd.
|
2025-08-22 | |
| $38.00/5mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-05 |








 China
China