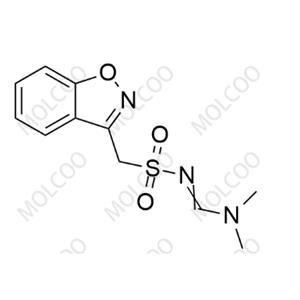
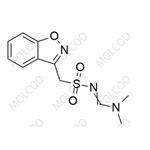
Product Details
| Product Name: Zonisamide USP Related Compound C | CAS No.: 1217201-89-2 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000000 | Release date: 2025/07/31 |
Zonisamide USP Related Compound C 1217201-89-2
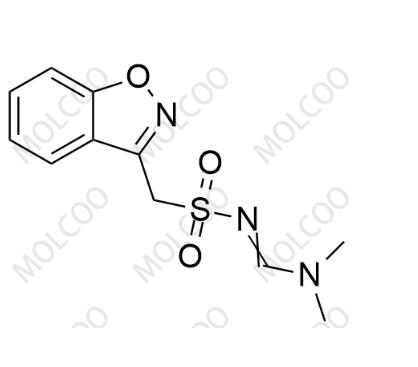

Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-22 | |
| $32.00/1mg |
VIP4Y
|
TargetMol Chemicals Inc.
|
2025-10-25 | |
| $50.00/1kg |
VIP3Y
|
Zibo Hangyu Biotechnology Development Co., Ltd
|
2023-11-07 |
INQUIRY








 China
China