2-Allylphenol: Antifungal Activity & Anti-Inflammatory Properties
Nov 13,2025
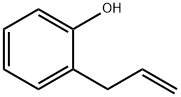
2-allylphenol is defined as a phenolic compound characterized by an allyl group attached to the para position of the hydroxyl-bearing aromatic ring, and it is involved in oxidative cyclization reactions, particularly in the context of palladium(II) complex catalysis.

Antifungal Activity of 2-Allylphenol Derivatives on the Botrytis cinerea Strain
The fungus Botrytis cinerea, responsible for the so-called gray mold disease, is a phytopathogen that causes serious damage to the agricultural industry, mainly due to its ability to infect various important crops, as well as its tendency to generate resistance to synthetic fungicides used to control this infection. The antifungal effects of these compounds on phytopathogenic fungi have also been assessed. Eugenol and its hemisynthetic derivatives present activity on the mycelial growth of a virulent and multiresistant isolate of B. cinerea (PN2), with IC50 values that vary between 31 and 95 μg mL−1. Recently, 2-allylphenol and some of its derivatives were tested against Phytophthora cinnamomi. Interestingly, the antifungal activity is enhanced in those compounds where a nitro group is attached in the para position relative to the hydroxyl group. Additionally, several of these 2-allylphenol derivatives exhibit antifungal activities similar to or better than metalaxyl®, a commercial fungicide commonly used to control P. cinnamomic.[1]
Derivatives of 2-allylphenol were obtained by either attaching electron-attracting groups (-NO2) to the aromatic ring in ortho and para positions, relative to the hydroxyl group, or converting -OH into methoxy or acetyl groups. Results indicate that the IC50 varies from 1.00 to 136 μg mL−1, and the value obtained for 2-allylphenol (68.0 μg mL−1) is in the same range as that previously reported (48.8 μg mL−1) for the inhibition of B05.10, a nonresistant strain of B. cinerea. Thus, the data indicate that growth inhibition of B. cinerea, evaluated as IC50 values, is clearly dependent on the chemical structure of 2-allylphenol derivatives. The synthesis and characterization of this compound are described below. IR spectra were obtained in a Thermo Scientific Nicolet Impact 6700 FT-IR spectrometer (San Jose, CA, USA) using KBr pellets, and the frequencies were reported in cm−1. 1H and 13C-NMR (DEPT 135 and DEPT 90) were performed on a Bruker Avance NEO 400 Digital NMR spectrometer (Bruker, Rheinstetten, Germany), operating at 400.1 MHz for 1H and 100.6 MHz for 13C.
2-Allylphenol Reduces IL-1β and TNF-α
Nociception is the transmission of signals captured by free nerve endings in response to noxious stimuli; this stimulus is converted into electrical signals that reach the spinal cord, thalamus, and cerebral cortex. 2-Allylphenol (2-AP) is a synthetic phenylpropanoid, structurally related to cardanol, thymol, and ortho-eugenol. It is a widely marketed fungicide in China under the name Yinguo, where studies attribute its activity by inducing cyanide-resistant respiration, causing an ATP decrease, and inhibiting the fungal pathogen respiration. However, 2-Allylphenol has no other properties described in the literature, making this study relevant to investigate its capacity to reduce pain, inflammation, and oxidative stress. Due to the similarity between 2-AP and other bioactive phenylpropanoids, the present research aims at evaluating the antioxidant potential of 2-allylpheno, which was determined in vitro through total antioxidant capacity, DPPH (1,1-diphenyl-2-picrylhydrazyl) sequestering activity, hydroxyl radical sequestering activity, and superoxide sequestering activity tests; at elucidating its antinociceptive activity, which was performed in vivo experimental models of acetic acid induced abdominal contortions, glutamate test, and formalin-induced paw-licking test.[2]
2-AP presents antinociceptive activity via adenosinergic system participation. The anti-inflammatory properties of 2-Allylphenol involve TNF-α and IL-1β reductions, being relevant data that corroborate its antinociceptive effect. 2-AP was able to sequestrate the superoxide radical, demonstrating its antioxidant activity. Docking, interactions between 2-Allylphenol and critical residues present in the active site of the A2a adenosine receptor were identified, suggesting agonistic action for this receptor subtype. Given our results, the clinical potential of 2-allylpheno to treat pain and inflammation is evident.
References
[1]Olea AF, Rubio J, Sedan C, Carvajal D, Nu?ez M, Espinoza L, Llovera L, Nu?ez G, Taborga L, Carrasco H. Antifungal Activity of 2-Allylphenol Derivatives on the Botrytis cinerea Strain: Assessment of Possible Action Mechanism. Int J Mol Sci. 2023 Mar 31;24(7):6530. doi: 10.3390/ijms24076530. PMID: 37047503; PMCID: PMC10095406.
[2]Arag?o Neto HC, da Fonsêca DV, Braga RM, Scotti MT, do Nascimento TWAB, Assis DB, Rodrigues-Mascarenhas S, Silva LHAC, Galv?o JGFM, Rocha HAO, Vidal AAJ, Filho JMB, de Almeida RN. 2-Allylphenol Reduces IL-1β and TNF-α, Promoting Antinociception through Adenosinergic, Anti-Inflammatory, and Antioxidant Mechanisms. Oxid Med Cell Longev. 2019 Mar 31;2019:1346878. doi: 10.1155/2019/1346878. PMID: 31049124; PMCID: PMC6462329.
- Related articles
- Related Qustion
2-Coumaranones have a mechanism involving deprotonation and 1,2-dioxetanone, with versatile applications and facile synthesis.....
Nov 13,2025Organic ChemistryStigmasterol (plant-derived phytosterol) regulates lipid metabolism, and inhibits triple-negative breast cancer stem cells by suppressing JAK3.....
Nov 13,2025API2-Allylphenol
1745-81-9You may like
- 2-Allylphenol
-

- $0.00 / 1kg
- 2025-11-21
- CAS:1745-81-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000
- 2-Allylphenol
-

- $10.00 / 1KG
- 2025-11-14
- CAS:1745-81-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- 2-Allylphenol
-

- $95.00 / 500mg
- 2025-10-24
- CAS:1745-81-9
- Min. Order:
- Purity: 99.74%
- Supply Ability: 10g