Is calcium carbonate easily soluble in water? What are its applications?
Mar 21,2024
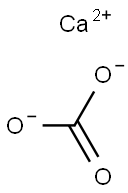
Calcium carbonate, with the chemical formula CaCO₃, is an inorganic compound that serves as a primary component of limestone, marble, and other minerals. It is essentially insoluble in water but can dissolve in hydrochloric acid.

Calcium carbonate is a common substance on Earth, found in minerals like aragonite, calcite, chalk, limestone, marble, and travertine, as well as being a major constituent of certain animal bones and shells. Widely used in various industries, calcium carbonate is an important construction material.
Main types of calcium carbonate
There are two main types of calcium carbonate: heavy calcium carbonate and light calcium carbonate.
Heavy calcium carbonate, also known as ground calcium carbonate, is produced by mechanically crushing natural materials such as calcite, limestone, chalk, or shells.
Light calcium carbonate, also referred to as precipitated calcium carbonate, is created by calcining raw materials like limestone to produce lime and carbon dioxide, hydrating the lime to form slaked lime, and then carbonating the slaked lime with carbon dioxide to precipitate calcium carbonate, which is further dehydrated, dried, and powdered.
Application fields of calcium carbonate
Applications of calcium carbonate span various industries, including rubber, plastics, papermaking, coatings, and inks, where it is utilized as a filler. It finds extensive use in organic synthesis, metallurgy, glass manufacturing, asbestos production, among other industrial processes. Calcium carbonate is employed as a neutralizing agent for industrial wastewater, an acid reducer for gastric and duodenal ulcers, a detoxifying agent for acidosis, an SO₂ eliminator in exhaust gases, a feed additive for dairy cattle, and an anti-adhesive agent in oil felt. Moreover, it serves as a raw material in tooth powder, toothpaste, and other cosmetic products.
- Related articles
- Related Qustion
- Lewis Structure of Calcium Carbonate and the Reaction with HCl May 7, 2025
Calcium Carbonate is an ionic compound in which cation is Ca2+ and anion is CO32-. This article will introduce its lewis structure and the reaction with HCl.
- Benefits and side effects of Calcium carbonate supplements Dec 26, 2024
Calcium carbonate is a nutritional supplement used to supplement calcium. Calcium is an essential nutrient for normal growth and development.
- Is daily consumption of calcium carbonate considered safe? Aug 26, 2024
Calcium carbonate is generally safe for daily calcium supplementation.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAcetylene is dimerized in the presence of hydrochloric acid solution of cuprous chloride and ammonium chloride to obtain vinylacetylene.....
Mar 21,2024APICalcium carbonate
471-34-1You may like
Calcium carbonate manufacturers
- Calcium carbonate
-
- 2025-12-20
- CAS:471-34-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- LIGHT CALCIUM CARBONATE
-

- $15.00 / 25kg
- 2025-12-19
- CAS:471-34-1
- Min. Order: 1000kg
- Purity: 98%
- Supply Ability: 20 tons
- Calcium carbonate
-

- $0.00 / 1kg
- 2025-12-19
- CAS:471-34-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT