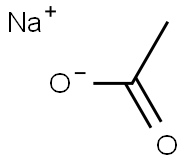
Is Sodium acetate an acid or a base salt?
Jul 16,2024
Sodium acetate (CH3COONa) is in some cases a basic salt. CH3COONa is produced by the reaction of acetic acid with sodium hydroxide and it is the salt of the acetate ion. According to Lowry -Bronsted acid base theory, an acid is a substance that tends to give protons to any other substance, while a base is defined as a substance that tends to receive protons from other substances. Acids and bases interact to produce an ionic compound known as a salt. Whereas acetic acid has a dissociation constant equal to 1.8 × 10-5 and is a weak acid, sodium hydroxide is a strong base. Acids have conjugate bases. The weak base acetic acid loses its proton to form the conjugate base acetate ion. This is a stronger base. It is a conjugate base of a weak acid. It is a stronger base. Hence, we can say that sodium acetate is a basic salt.

- Related articles
- Related Qustion
- Effect of short-term feeding of sodium acetate on milk fat yield in dairy cows Dec 19, 2023
Supplementation with sodium acetate (NaAcet) increases milk fat production through an apparent stimulation of de novo lipogenesis in the mammary gland.
- Sodium Acetate: Preparation, Reactions and Applications Apr 12, 2023
Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
- Practical Applications of Sodium Acetate Nov 12, 2019
Sodium acetate salt, or simply sodium acetate, has many practical uses. It is the conjugate base of a weak acid, meaning that it only partially ionizes when dissolved in water.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIL-Tyrosine is the left-handed isomer of the aromatic amino acid tyrosine. It is a naturally occurring tyrosine that is synthesised in the body from L-phenylalanine.....
Dec 16,2024Amino Acids and ProteinsSodium acetate
127-09-3You may like
- Sodium acetate
-
- 2025-07-08
- CAS:127-09-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sodium acetate anhydrous
-
- $10.00 / 1kg
- 2025-07-08
- CAS:127-09-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100kg
- Sodium acetate
-

- $29.00 / 1g
- 2025-07-07
- CAS:127-09-3
- Min. Order:
- Purity: ≥98%
- Supply Ability: 10g