The pharmacological research of sodium octanoate
Jul 1,2025
Introduction
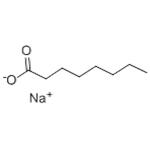
Sodium octanoate (SO;Figure 1), a medium chain monocarboxylic saturated fatty acid, commonly found in dairy products and coconut oils, has shown promising therapeutic potential in the treatment of various diseases. Several in vitro and in vivo studies have suggested that SO may provide therapeutic benefits by mitigating oxidative stress, inflammation, and apoptosis, as well as stabilizing mitochondria, particularly in the context of ischemia-reperfusion injury (IRI).[1]Sodium octanoate is highly hydrophilic and is characterized by its rapid generation of metabolic energy. Studies have demonstrated that intraperitoneal administration of sodium octanoate post-myocardial infarction in mice can significantly reduce infarct size and enhance cardiac function, potentially through the promotion of antioxidant gene expression and the inhibition of cardiomyocyte apoptosis. In the context of sepsis, octanoic acid has been shown to improve lipid metabolism in lipopolysaccharide (LPS)-treated mice , attenuate LPS-induced intestinal injury, and ameliorate acute liver injury. Emerging evidence from in vitro and in vivo studies suggests that octanoic acid or sodium octanoate may exert therapeutic effects by reducing tissue damage and protecting vital organs such as the heart and brain, via mechanisms that include the alleviation of inflammation , mitigation of oxidative stress injury, inhibition of apoptosis, and stabilization of mitochondrial function.[2]

1. alleviate cardiac and cerebral injury in porcine
Traumatic cardiac arrest (TCA) is a severe condition with a high mortality rate, and patients whosurvive from TCA face a poor prognosis due to post-resuscitation injury, including cardiac and cerebral injury,which remains a serious challenge. Sodium octanoate has shown protective effects against various diseases.The present study aims to investigate sodium octanoate's protective effects against cardiac and cerebral injury after TCA in a porcine model. The study included a total of 22 male domestic pigs divided into three groups: Sham group (n=7), TCA group (n=7), and sodium octanoate (SO) group (n=8). Hemorrhage was initiated via the right femoral artery by a blood pump at a rate of 2 ml/kg/min to establish TCA model. The Sham group underwent only endotracheal intubation and arteriovenous catheterization, without experiencing the blood loss/cardiac arrest/resuscitation model. At 5 min after resuscitation, the SO group received a continuous sodium octanoate infusion while the TCA group received the same volume of saline. General indicators were monitored, and blood samples were collected at baseline and at different time points after resuscitation. At 24 h after resuscitation, pigs were sacrificed, and heart and brain were obtained for cell apoptosis detection, iron deposition staining, oxidative stress detection, and the expression of ferroptosis-related proteins (ACSL4 and GPX4).Sodium octanoate significantly improved mean arterial pressure, cardiac output and ejection fraction induced by TCA. Serum biomarkers of cardiac and cerebral injury were found to increase at all time points after resuscitation, while sodium octanoate significantly reduced their levels. The apoptosis rates of cardiomyocytes and cerebral cortex cells in the SO group were significantly lower than in the TCA group, along with a reduced area of iron deposition staining. The sodium octanoate also reduced oxidative stress and down-regulated ferroptosis which was indicated by protein level alteration of ACSL4 and GPX4. This study's findings suggest that early infusion of sodium octanoate significantly alleviates post-resuscitation cardiac and cerebral injury in a porcine model of TCA, possibly through inhibition of cell apoptosis and GPX4-mediated ferroptosis. Therefore, sodium octanoate could be a potential therapeutic strategy for patients with TCA.[1]
2.Protective effects against SIMD
This study aims to evaluate the therapeutic effects of sodium octanoate on sepsis-induced myocardial dysfunction (SIMD) in a murine model and to explore its underlying mechanisms. Male mice were subjected to sepsis models through two methods: intraperitoneal injection of lipo-polysaccharide (LPS) and cecal ligation and punction (CLP). Mice received interval doses of sodium octanoate every 2 hours or4 hours for a total of six times or three times after LPS treatment. The relationship between sodium octanoate and G protein coupled receptor 84 (GPR84) was evaluated through GEO data analysis and molecular docking studies. DBA/2mice were used to study the role of the GPR84 protein in the sodium octanoate-mediated protection. Energy metabolomics was utilized to comprehensively assess the impact of sodium octanoate on the levels of cardiac energy metabolic products in septic mice. Histone modification identification techniques were used to further identify the specific sites of histone modification in the hearts of sodium octanoate-treated septic mice. Sodium octanoate treatment significantly improved myocardial contractile function, restored the oxidative stress imbalance and enhanced the myocardium's resistance to oxidative injury. Sodium octanoate significantly promotes the expression of GPR84. The loss of GPR84 function markedly attenuates the protective effects of sodium octanoate. Sodium octanoate enhancedmyocardial energy metabolism by promoting the synthesis of acetyl-CoA and upregulating genes involved inf atty acid β-oxidation which were abolished by medium-chain acyl-CoA dehydrogenase (MCAD) knockdown. Sodium octanoate induced histone acetylation, particularly at H3K123 and H3K80. This study demonstrates that sodium octanoate exerts protective effects against SIMD through both GPR84-mediatedanti-inflammatory and antioxidant actions and GPR84-independent enhancement of myocardial energy metabolism, possibly mediated by MCAD.[2]
3.Modulates the Innate Immune Response
Bovine mammary epithelial cells (bMECs) contribute to mammary gland defense against invading pathogens, such as Staphylococcus aureus (intracellular facultative), which is recognized by TLR2. In a previous report, sodium octanoate could induce (0.25 mM) or inhibit (1 mM) S. aureus internalization into bMECs and differentially regulates the innate immune response (IIR). The results showed that α5β1 integrin membrane abundance (MA) was increased in 0.25 mM sodium octanoate-treated cells, but TLR2 or CD36 MA was not modified. When these receptors were blocked individually, 0.25 mM sodium octanoate-increased S. aureus internalization was notably reduced. Interestingly, in this condition, the IIR of the bMECs was impaired because MAPK (p38, JNK, and ERK1/2) phosphorylation and the activation of transcription factors related to these pathways were decreased. In addition, the 1 mM sodium octanoate treatment induced TLR2 MA, but neither the integrin nor CD36 MA was modified. The reduction in S. aureus internalization induced by 1 mM sodium octanoate was increased further when TLR2 was blocked. In addition, the phosphorylation levels of the MAPKs increased, and 13 transcriptional factors related to the IIR were slightly activated (CBF, CDP, c-Myb, AP-1, Ets-1/Pea-3, FAST-1, GAS/ISRE, AP-2, NFAT-1, OCT-1, RAR/DR-5, RXR/DR-1, and Stat-3). Moreover, the 1 mM sodium octanoate treatment up-regulated gene expression of IL-8 and RANTES and secretion of IL-1β. Notably, when 1 mM sodium octanoate-treated bMECs were challenged with S. aureus, the gene expression of IL-8 and IL-10 increased, while IL-1β secretion was reduced. In conclusion, our results showed that α5β1 integrin, TLR2 and CD36 are involved in 0.25 mM SO-increased S. aureus internalization in bMECs. In addition, 1 mM sodium octanoate activates bMECs via the TLR2 signaling pathways (p38, JNK, and ERK1/2), which improves IIR before S. aureus invasion. Additionally, sodium octanoate (1 mM) might exert anti-inflammatory effects after bacterial internalization.[3]
Conclusion
Further research is warranted to investigate the therapeutic potential of sodium octanoate in critical and severe illnesses. This should encompass molecular biology studies, the use of large animal models, and a gradual assessment of the feasibility and efficacy of sodium octanoate in clinical settings.
References
1. Lin Y, Chen Q, Zhang G, et al. Sodium octanoate alleviates cardiac and cerebral injury after traumatic cardiac arrest in a porcine model. Am J Emerg Med. 2024;78:48-56. doi:10.1016/j.ajem.2023.12.048
2. Lin Y, Zhang W, Jiang X, et al. Sodium octanoate mediates GPR84-dependent and independent protection against sepsis-induced myocardial dysfunction. Biomed Pharmacother. 2024;180:117455. doi:10.1016/j.biopha.2024.117455
3. Alva-Murillo N, Ochoa-Zarzosa A, López-Meza JE. Sodium Octanoate Modulates the Innate Immune Response of Bovine Mammary Epithelial Cells through the TLR2/P38/JNK/ERK1/2 Pathway: Implications during Staphylococcus aureus Internalization. Front Cell Infect Microbiol. 2017;7:78. Published 2017 Mar 15. doi:10.3389/fcimb.2017.00078
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering2,2-Bis(3-amino-4-hydroxyphenyl)hexafluorop forms polyimide membranes with gas selectivity and proton conductivity for fuel cells/microfiltration.....
Jul 1,2025Chemical ReagentsSodium octanoate
1984-06-1You may like
Sodium octanoate manufacturers
- Sodium octanoate
-

- $0.00 / 25Kg/Drum
- 2025-12-19
- CAS:1984-06-1
- Min. Order: 1KG
- Purity: 99%-101%
- Supply Ability: 1000KGS
- Sodium octanoate
-

- $0.00 / 25kg
- 2025-12-19
- CAS:1984-06-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1
- Sodium octanoate
-
- 2025-12-19
- CAS:1984-06-1
- Min. Order:
- Purity: 0.99
- Supply Ability:






