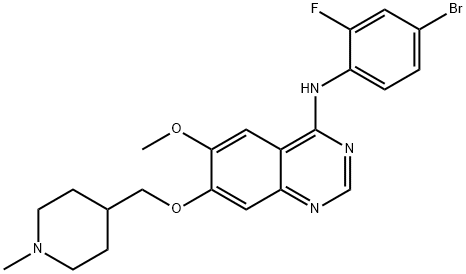
Vandetanib: Efficacy, Safety and Optimal Dosing in Medullary Thyroid Cancer
May 14,2025
Vandetanib is in a class of medications called kinase inhibitors.It works by blocking the action of an abnormal protein that signals cancer cells to multiply.This helps slow or stop the spread of cancer cells.

Efficacy and Safety of Vandetanib in Progressive and Symptomatic Medullary Thyroid Cancer
Hereditary MTC is a component of multiple endocrine neoplasia (MEN) types 2A (including familial MTC) and 2B. Virtually all patients with MEN2A or MEN2B have germline mutations in the RET proto-oncogene, and a high percentage of sporadic MTCs have somatic RET mutations at diagnosis. Therefore, targeting patients with RET-positive status and MTC has a therapeutic potential. The only other driver point-mutations found in MTC are RAS mutations. Vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) also contribute to growth and invasiveness of MTC.Vandetanib is a once-daily, oral TKI that acts by targeting several cell receptors in combination, including those involved in RET, VEGFR2, and EGFR signaling. Vandetanib was approved in the United States for the treatment of symptomatic or progressive MTC in patients with unresectable locally advanced or metastatic disease. In Japan, vandetanib was approved for unresectable MTC on the basis of findings of the phase III ZETA (Zactima Efficacy in Thyroid Cancer Assessment) trial. In the European Union, approval was granted for treatment of aggressive and symptomatic advanced (unresectable locally advanced or metastatic disease) MTC. Patients with progressive and symptomatic advanced MTC represent a subset of the ZETA trial cohort with a strong medical need, and it is of interest to explore the clinical benefits of vandetanib treatment in patients with differing levels of disease.[1]
A total of 331 patients with unresectable locally advanced or metastatic MTC were randomly assigned in a 2:1 ratio to receive oral vandetanib at a starting dose of 300 mg/day (n = 231) or placebo (n = 100) until disease progression occurred. Upon objective disease progression, on the basis of investigator assessment, patients discontinued the study drug and were unblinded. The choice to offer patients open-label vandetanib was based on local site review. Those with post-progression status could elect to enter open-label treatment with vandetanib until a withdrawal criterion was met. In addition, patients determined by central review not to have progressive disease also had the option to be unblinded and receive open-label medication. All patients were observed for survival. The patients signed an informed consent to enter the study. For the primary ZETA analysis, data from the central read, which was not conducted in real time, were used in preference to data from the local site review. However, the choice to offer the patients open-label vandetanib was based on local site review. This resulted in patients not progressive by central review receiving open-label medication. To exclude this source of potential bias and to avoid it in the current post hoc analysis, local investigator-assessed progression was used to determine PFS. Additional details can be found in the Discussion. Kaplan-Meier survival curves for PFS, OS, and TWP were compared for the vandetanib and placebo arms in each of the 4 disease severity subgroups using log-rank tests. No statistical comparisons were made between disease severity subgroups for treatment outcomes. Hazard ratios (HRs) for PFS, OS, and TWP in the subgroups were estimated using Cox proportional hazards regression. The ORR within each disease severity subgroup was summarized as the number and percentage of patients experiencing objective response.
Vandetanib showed statistically significant prolonged median PFS in both the progression and symptoms subgroup and the symptoms-only subgroup, compared with placebo, but not in the progression-only and no symptoms/no progression subgroups. It should be noted that the number of patients in the symptoms only and the no progression/no symptoms subgroups, both in the vandetanib and placebo arms, was low and that these results should be interpreted with caution. The fact that OS was not significantly different between vandetanib- and placebo-treated patients in any of the subgroups may be due to a treatment effect from participation in the open-label arm for this outcome measure. The ORR was higher in patients treated with vandetanib than placebo in each disease severity subgroups. Although objective responses were observed in all severity subgroups, no comparisons were made between the severity subgroups, because the number of patients in the symptoms-only group and the no progression/no symptoms group was very small. Previous studies of TKIs that included patients with MTC with documented disease progression and symptoms at baseline have reported an overall ORR of 28% while on treatment. In this study, 37.0% percent of the subgroup with progression and symptoms, and 47.6% of patients with progression only, had an objective response during randomly assigned treatment with vandetanib.
Safety and efficacy of two starting doses of vandetanib in advanced medullary thyroid cancer
Vandetanib (Caprelsa) is a once-daily, oral tyrosine kinase inhibitor that selectively targets rearranged during transformation (RET) receptor, vascular endothelial growth factor receptor and epidermal growth factor receptor signaling. In the Phase III ZETA clinical trial in patients with locally advanced or metastatic hereditary MTC, median progression-free survival was significantly prolonged from 19.3 months with placebo to a predicted 30.5 months (median not yet reached at data cut-off) with vandetanib (hazard ratio, 0.46; P < 0.0001). Based on these results, the US Food and Drug Administration (FDA) approved vandetanib for the treatment of symptomatic or progressive MTC in patients with unresectable locally advanced or metastatic disease in 2011.A key issue relating to the use of vandetanib is whether the Phase III study identified the correct dose of vandetanib given several unexplained deaths in the vandetanib treatment group. To address this concern and to help inform decision-making on vandetanib starting doses, this study was designed to identify the objective response rates (ORR) and evaluate the safety and tolerability of two starting doses of vandetanib (150 and 300 mg) in patients with symptomatic or progressive MTC.[2]
Patients with advanced or metastatic MTC have a poor prognosis, with chemotherapy and radiation therapy being largely ineffective. Increased understanding of the molecular pathogenesis of MTC has resulted in the development of targeted therapies and represents an important advance for clinical management of this disease. In 2011, the US FDA approved vandetanib for the treatment of symptomatic or progressive MTC in patients with unresectable locally advanced or metastatic disease. In the EU, vandetanib was approved by the EMA in 2012, for the treatment of aggressive and symptomatic MTC in patients with unresectable locally advanced or metastatic disease. Assessment of the benefit‒risk relationship of different vandetanib starting doses has not previously been performed. This may be especially important for populations that are less able to tolerate treatment-related side effects, particularly given the potential for long-term vandetanib treatment. We therefore set out to investigate the safety and efficacy of two vandetanib starting doses, 150 and 300 mg, in patients with unresectable locally advanced or metastatic MTC with progressive or symptomatic disease.
To conclude, in the absence of a curative treatment for locally advanced or metastatic MTC, the availability of treatments with the ability to substantially prolong time to disease progression with limited side effects is an important clinical advance for this disease. Although the results of our study showed clinical response with both vandetanib doses, the 300 mg dose showed a more favorable trend compared with the 150 mg group in ORR. This indicates that, for most patients, 300 mg vandetanib is the most appropriate starting dose; however, dose reductions to manage AEs and lower initial doses for patients with particular comorbidities can be considered.
References
[1]Kreissl MC, Bastholt L, Elisei R, Haddad R, Hauch O, Jarząb B, Robinson B, Colzani R, Foster M, Weiss R, Schlumberger M. Efficacy and Safety of Vandetanib in Progressive and Symptomatic Medullary Thyroid Cancer: Post Hoc Analysis From the ZETA Trial. J Clin Oncol. 2020 Aug 20;38(24):2773-2781. doi: 10.1200/JCO.19.02790. Epub 2020 Jun 25. PMID: 32584630; PMCID: PMC7430220.
[2]Hu, Mimi I et al. “Safety and efficacy of two starting doses of vandetanib in advanced medullary thyroid cancer.” Endocrine-related cancer vol. 26,2 (2019): 241-250. doi:10.1530/ERC-18-0258
- Related articles
- Related Qustion
- What to know when taking Vandetanib? Mar 24, 2025
Vandetanib works by blocking the action of abnormal proteins that signal cancer cells to multiply, thereby helping to slow or stop the spread of cancer cells.
- Vandetanib- Application in Cancer Therapy Dec 10, 2019
Vandetanib is a multiple signal transduction inhibitor including the RET tyrosine kinase, epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF) receptor (VEGFR), ERK and with antiangiogenic activity.
In early in vitro studies, the mechanism of action of equol was found to indicate that the compound may be associated with cancer risk.....
May 13,2025API2-Amino-5-methylpyridine is an important intermediate which is widely used in medicine, dye and pesticide industries. Here mainly introduces other properties.....
May 14,2025Organic Synthesis IntermediateVandetanib
443913-73-3You may like
- Vandetanib
-

- $0.00 / 1KG
- 2025-07-07
- CAS:443913-73-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS
- Vandetanib
-
- $45.00 / 10mg
- 2025-07-04
- CAS:443913-73-3
- Min. Order:
- Purity: 99.84%
- Supply Ability: 10g
- Vandetanib
-

- $0.00 / 1KG
- 2025-07-04
- CAS:443913-73-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month