3-Hydroxy-4-methyl-5-nitrobenzoic acid methyl ester synthesis
- Product Name:3-Hydroxy-4-methyl-5-nitrobenzoic acid methyl ester
- CAS Number:125229-13-2
- Molecular formula:C9H9NO5
- Molecular Weight:211.17
Yield:-
Reaction Conditions:
with thionyl chloride at 0;Reflux;
Steps:
1F.4
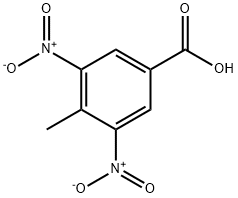
Step 4:; At 0-50C, to a solution of 3-hydroxy-4-methyl-5-nitrobenzoic acid (28 g, 141 mmole) in methanol (280 ml), thionyl chloride (15.5ml, 212 mmole) was added dropwise over a period of 30 minutes. After the addition, the reaction mixture was brought to room temperature and then refluxed for 4 h. The reaction mixture was concentrated under vacuum. The resulting solid residue was dissolved in ethyl acetate (500 ml) and washed with sodium bicarbonate solution. Ethyl acetate extract was dried over Na2SO4 and concentrated under vacuum to yield 30 g of methyl 3-hydroxy-4-methyl-5-nitrobenzoate as colorless solid (quantitative). The crude product was used as such for the next step without characterization.
References:
WO2010/59549,2010,A1 Location in patent:Page/Page column 26-28
16533-71-4
220 suppliers
$9.00/10g

125229-13-2
12 suppliers
inquiry

1057652-89-7
11 suppliers
inquiry

125229-13-2
12 suppliers
inquiry

49592-71-4
40 suppliers
inquiry

125229-13-2
12 suppliers
inquiry

72922-60-2
29 suppliers
inquiry

125229-13-2
12 suppliers
inquiry