
1H-indazole-3-carboxylic acid methyl ester synthesis
- Product Name:1H-indazole-3-carboxylic acid methyl ester
- CAS Number:43120-28-1
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
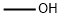
67-56-1
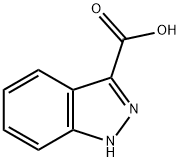
4498-67-3

43120-28-1
Indazole-3-carboxylic acid (5.0 g, 30.8 mmol) was dissolved in methanol (50 mL) at 0 °C and thionyl chloride (15 mL) was added slowly and dropwise. After the dropwise addition was completed, the reaction mixture was heated to reflux temperature and maintained at this temperature for 1.5 hours. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure to give the crude product. The crude product was neutralized by adding saturated sodium bicarbonate solution (50 mL) to the crude product and subsequently extracted with ethyl acetate (50 mL x 3). The organic phases were combined and dried over anhydrous sodium sulfate. The desiccant was removed by filtration and the filtrate was concentrated under reduced pressure to afford methyl 1H-indazole-3-carboxylate as a white solid (5.1 g, 94% yield). The NMR hydrogen spectrum (300 MHz, d6-DMSO) data were as follows: δ 13.91 (s, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.44 (ddd, J = 8.3 Hz, 6.9 Hz, 1.1 Hz, 1H), 7.30 (dd, J = 7.9 Hz, 6.9 Hz, 0.9 Hz, 1H), 3.92 (s, 3H).
Yield:43120-28-1 93%
Reaction Conditions:
Stage #1: methanol;2H-indazole-3-carboxylic acid;sulfuric acid for 16 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
1; A
A solution of 2H-indazole-3-carboxylic acid (5.00 g, 30.8 mmol) and concentrated sulphuric acid (0.16 mL, 3.08 mmol) in methanol (100 mL) was heated to reflux for 16 hours. The reaction mixture was concentrated in vacuo and then partitioned between ethyl acetate and water, washed with saturated sodium bicarbonate solution (aq.), the aqueous phase extracted with ethyl acetate, the combined organic layers washed with brine, dried (magnesium sulfate) and concentrated to give 2.02 g (93%) of the title compound.
References:
US2010/305120,2010,A1 Location in patent:Page/Page column 43
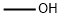
67-56-1
790 suppliers
$9.00/25ml

4498-67-3
487 suppliers
$6.00/5g

43120-28-1
173 suppliers
$10.00/1g

35613-44-6
94 suppliers
$35.00/250mg

43120-28-1
173 suppliers
$10.00/1g

4498-67-3
487 suppliers
$6.00/5g

43120-28-1
173 suppliers
$10.00/1g

