
2,2',7,7'-Tetrabromo-9,9'-spirobifluorene synthesis
- Product Name:2,2',7,7'-Tetrabromo-9,9'-spirobifluorene
- CAS Number:128055-74-3
- Molecular formula:C25H12Br4
- Molecular Weight:631.98
![9,9'-Spirobi[9H-fluorene]](/CAS/GIF/159-66-0.gif)
159-66-0

128055-74-3
General procedure for the synthesis of 2,2',7,7'-tetrabromo-9,9'-spirobifluorene from 9,9'-spirobifluorene: 80 mg (0.5 mmol) of anhydrous FeCl3 was added to 30 ml of dichloromethane solution containing 3.16 g (10.0 mmol) of 9,9'-spirobifluorene. A solution of 2.1 ml (41 mmol) of bromine dissolved in 5 ml of dichloromethane was added slowly and dropwise over 10 min. The reaction mixture was refluxed for 6 hours. After completion of the reaction, it was cooled to room temperature and the product precipitated as a precipitate. The precipitate was collected by diafiltration and washed with a small amount of cold dichloromethane. Drying gave 6.0 g (95% yield) of the target product 2,2',7,7'-tetrabromo-9,9'-spirobifluorene as a white solid.

171408-84-7
216 suppliers
$8.00/1g

128055-74-3
219 suppliers
$17.00/1g
Yield:128055-74-3 85%
Reaction Conditions:
with bromine in chloroform at 0; for 3 h;
Steps:
1.3
3) Synthesis of a compound 3 The compound 2 (10.6 mmol, 5 g) was dissolved in 150 ml of chloroform and cooled to 0°C, bromine (21.19 mmol, 3.39 g) was slowly dropped thereon, and agitation was conducted for 3 hours. After the reaction, 50 ml of 2 M potassium hydroxide was injected into the reaction vessel to bring about neutralization. The reactants were then washed three times using distilled water. Next, an organic layer was separated and subjected to vacuum distillation, and the resulting solid was recrystallized using a mixed solution of chloroform and ethanol to produce a compound 3 (9.01 mmol, 5.65 g) (Yield 85 %).
References:
EP1777227,2007,A1 Location in patent:Page/Page column 6-7
![9,9'-Spirobi[9H-fluorene]](/CAS/GIF/159-66-0.gif)
159-66-0
220 suppliers
$9.00/1g

128055-74-3
219 suppliers
$17.00/1g
![2-Bromo-9,9'-spirobi[9H-fluorene]](/CAS/GIF/171408-76-7.gif)
171408-76-7
267 suppliers
$8.00/1g

128055-74-3
219 suppliers
$17.00/1g

67665-44-5
1 suppliers
inquiry

128055-74-3
219 suppliers
$17.00/1g
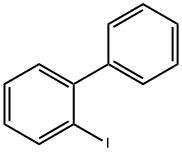
2113-51-1
258 suppliers
$6.00/1g

128055-74-3
219 suppliers
$17.00/1g