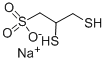
2,3-Dimercaptopropanesulfonic acid sodium salt synthesis
- Product Name:2,3-Dimercaptopropanesulfonic acid sodium salt
- CAS Number:4076-02-2
- Molecular formula:C3H7NaO3S3
- Molecular Weight:210.27
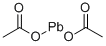
301-04-2

51116-03-1

4076-02-2
1. Preparation of sodium hydrosulfide: 3250 mL of anhydrous ethanol was added to 5 L of high-pressure reactor, and 110 g (1.60 mol) of sodium ethoxide was added at once under stirring. After the addition was completed, the inlet was sealed, and the reaction temperature was controlled below 25 °C by jacketed cooling water. Under stirring, high-pressure hydrogen sulfide gas was introduced, and the reaction pressure was maintained in the range of 0.3-0.5 MPa for about 30 min until the solution pH reached 6.5-7.5. 2. Thiolation reaction: After completion of the reaction, release the pressure inside the kettle, open the lid, and add 230.3 g (0.75 mol) of sodium 2,3-dibromopropanesulfonate. Seal the lid of the kettle, control the internal temperature at 23-25 ℃, and pass the high-pressure hydrogen sulfide gas again under stirring, maintain the pressure at 0.3-0.5 MPa, and react for 90 min. After depressurization, the hydrogen sulfide gas was driven off by vacuum (the exhaust gas was absorbed by caustic solution and activated carbon in series). The reaction solution was transferred to another reaction flask, and the pH was adjusted to 4.6-4.7 by slowly adding 36% glacial acetic acid dropwise at room temperature, and the stirring was continued for 30 min until the pH stabilized. Subsequently at 10 ℃ below the stationary 4 hours, filtration to remove sodium acetate, to obtain the clarified thiolation reaction solution. 3. Lead salt reaction: 284.5 g of lead acetate trihydrate was dissolved in 625 mL of distilled water preheated to 50-55 °C. The reaction was carried out in the same manner as the reaction solution. Transfer the above thiolated reaction solution to a 5 L reaction flask, warm up to 50 °C with stirring, and slowly add the aqueous lead acetate solution. After addition, stirring was continued for 1 h. After aging for 2 h, it was filtered. The crude lead salt cake was washed with 50 °C hot water (800 mL + 600 mL + 400 mL) in three times, drained and washed with 1000 mL of hot water of the same temperature with stirring for 30 minutes. Finally, it was washed once with 400 mL of cold anhydrous ethanol with agitation, and dried under vacuum at 50 ℃ until constant weight, obtaining 264.7 g of 2,3-dimercaptopropanesulfonic acid lead salt complex, with a yield of 70.8% (in terms of sodium 2,3-dibromopropanesulfonate). 4. Lead desalting: 250 g of 2,3-Dimercaptopropanesulfonic acid lead salt complex was added into a 3 L reaction flask, 2000 mL of anhydrous ethanol was added, and hydrogen sulfide gas was slowly introduced under stirring at room temperature until the yellow lead salt particles completely disappeared and were transformed into black lead sulfide precipitate. After standing for 1 hour, the temperature was raised to 35°C to drive off the residual hydrogen sulfide gas. 60°C was heated and activated carbon was added, stirred to decolorize for 15 minutes, and filtered. Ground sodium bicarbonate powder was slowly added to the filtrate to adjust the pH to 4.3, and after neutralization, stirring was continued for 15 minutes until the pH was stable. After filtration, the filtrate was cooled and crystallized in a refrigerator below 1°C overnight. The crystals were filtered, washed once with cold anhydrous ethanol and dried under vacuum to obtain 97.8 g of crude sodium 2,3-dimercaptopropanesulfonate, with a content of 95.6% and a yield of 81.04% (in terms of lead salts). 5. Refining: 95.0 g of crude sodium 2,3-dimercaptopropanesulfonate was added into a reaction flask, 1300 mL of 90% ethanol was added, and the temperature was raised to 65-70 ℃ to make it completely dissolved. Powdered activated carbon was added, and the product was decolorized by stirring for 15 min and then filtered. The clarified filtrate was cooled and crystallized in a refrigerator at 10 °C overnight, filtered, washed and dried to obtain 78.8 g of white sodium 2,3-dimercaptopropanesulfonate finished crystals.
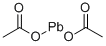
301-04-2
182 suppliers
$20.00/1G

51116-03-1
37 suppliers
$187.33/250mgs:

4076-02-2
200 suppliers
$45.00/100 mg
Yield:4076-02-2 78.8 g
Reaction Conditions:
Stage #1:sodium 2,3-dibromopropane-1-sulfonate with sodium ethanolate in ethanol at 23 - 25; under 2250.23 - 3750.38 Torr; for 1.5 h;
Stage #2:lead acetate in water at 50 - 55; for 1 h;Further stages;Reagent/catalyst;Temperature;
Steps:
1.2 Example 1 (Embodiment of the present invention)
1. Preparation of sodium hydrosulfide: put 3250ml of anhydrous ethanol into the 5L high pressure reactor, under stirring 110 g (1.60mol) of sodium ethoxide is added in one portion. After the addition, close and tighten the feeding port, control the internal temperature at below 25 °C passing through the jacket cooling water, under agitation passing high pressure hydrogen sulfide gas into the kettle through a hydrogen sulfide gas cylinder, the reaction pressure is stirred in the range of 0.3-0.5MPa for about 30 minutes, the pH of the solution is brought to a range of 6.5-7.5.2. Thiolation reaction: after the above reaction has been completed, the pressure in the kettle is released; open the kettle lid, one injection of 230.3 g (0.75mol) of sodium 2, 3-dibromo propanesulfonate. after the addition, tighten thekettle lid and control the internal temperature at 23 ° C ~ 25 ° C, under stirring the high pressure hydrogen sulfide gas is introduced into the kettle, carry on stirring reaction for 90 min when the pressure reaches 0.3~0.5Mpa. After decompressing, the hydrogen sulfide gas is vacuum-driven (the exhaust gas is absorbed in series by the caustic solution and the activated carbon), and the reaction liquid is pumped into another reaction bottle, and at room temperature slowly drop 36% glacial acetic acid to adjust the pH at 4.6~4.7 and continue stirring for 30min, until the pH value is retested. Then let stand for 4h below 10°C. Filtering off sodium acetate after that obtained clear thiolation reaction solution.3. Lead salt reaction: dissolve 284.5 g of lead acetate (trihydrate) into 625 ml of preheated at 50 ° C ~ 55 ° C distilled water. In addition, the above thiolation reaction solution is transferred into a 5L reaction flask, and the temperature is raised at 50 ° C, under stirring adding an aqueous solution of lead acetate into the reaction solution. After the addition, continue stirring for 1 h, and then aging for 2h, then filter to the mother liquor. The crude lead salt cake is washed 3 times with hot water at 50 °C (800ml+600ml+400ml), after draining, use 1000ml with the same temperature hot water to stir and wash 30min. Finally, it is stirred once with 400 ml of cold anhydrous ethanol and dried under vacuum at 50 ° C until get dry, and then obtained 264.7 g of 2, 3-dimercaptopropanesulfonic acid lead salt complex.Lead salt yield (same as sodium 2, 3-dibromopropane sulfonate, the following examples are the same): 70.8%.4. Lead removal, salt formation: 250g of 2, 3-dimercaptopropanesulfonic acid lead salt complex is put into a 3L reaction bottle, add 2000ml of anhydrous ethanol, and slowly pass hydrogen sulfide gas at room temperature with stirring, until the yellow 2,3-dimercaptopropanesulfonic acid lead salt all particles disappear completely and fully converted into black lead sulfide precipitate. After standing for 1 hour, the temperature is raised at 35 ° C to stir off the hydrogen sulfide gas. After that heat up at 60 ° C, adding activated carbon, stirring and de-coloring for 15 minutes, filtering, de-carbonization. Again under stirring the milled sodium bicarbonate powder is uniformly added into the filtrate, carefully neutralize at pΗ4.3. After the neutralization, continue stirring for 15 minutes, until the retest is unchanged, and then filter. The filtrate is placed in a freezer at 1 °C or below to cool and crystallize overnight. The crystals are filtered, washed once with cold anhydrous ethanol, dried and dried in vacuum to get dryness, and then obtained 97.8g of crude sodium 2, 3-dimercaptopropane sulfonate, the content is 95.6%. Based on lead salts, the yield is 81.04%.5. Refining: 95.0 g of crude sodium 2,3-dimercaptopropane sulfonate was put into the reaction flask.After adding 1300ml of 90% ethanol, the temperature is raised to 65 ° C ~ 70 ° C,After the total dissolution, the powdered activated carbon was added, and the mixture was decolorized by stirring for 15 minutes, and then filtered.The clarified filtrate was placed in a refrigerator at 10 ° C to cool and crystallize overnight, filtered, washed and dried according to law.78.8 g of white sodium 2,3-dimercaptopropanesulfonate finished crystals were obtained. Product purity and yield results are listed in the table below:
References:
Hefei Cube Pharmaceutical Co., Ltd.;Ji Junqiu;Gao Meihua;Li Xiaochang;Chen Jun CN102531981, 2016, B Location in patent:Paragraph 0034; 0036; 0092; 0095; 0097; 0102; 0104

51116-03-1
37 suppliers
$187.33/250mgs:

4076-02-2
200 suppliers
$45.00/100 mg