
2,3-dimethyl-6-nitro-2H-indazole synthesis
- Product Name:2,3-dimethyl-6-nitro-2H-indazole
- CAS Number:444731-73-1
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19

6494-19-5
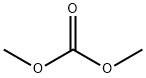
616-38-6

444731-73-1
General procedure for the synthesis of 2,3-dimethyl-6-nitroindazole (2a) from 3-methyl-6-nitro-1H-indazole (1a, 10.00 g, 56 mmol) and dimethyl carbonate (DMC, 6.04 g, 67 mmol): first, 3-methyl-6-nitro-1H-indazole and triethylenediamine (DABCO, 6.40 g, 56 mmol) were dissolved in 100 mL of N,N-dimethylformamide (DMF). The reaction mixture was stirred at room temperature for 15 min, followed by slow dropwise addition of dimethyl carbonate. After the dropwise addition, the reaction system was heated to reflux temperature with continuous stirring for 6 hours. Upon completion of the reaction, the mixture was cooled to room temperature, 120 mL of water was added and stirred for 15 minutes, at which time a large amount of light yellow solid precipitated. The solid product was collected by filtration and dried to give 2,3-dimethyl-6-nitroindazole (2a, 8.66 g, 81.1% yield). The melting point of the product was 187~187.6 °C. 1H NMR (400 MHz, DMSO-d6) δ: 2.67 (s, 3H), 4.14 (s, 3H), 7.73 (d, J = 9.2 Hz, 1H), 7.93 (d, J = 8.8 Hz, 1H), 8.51 (s, 1H).

6494-19-5
260 suppliers
$19.00/1g

616-38-6
722 suppliers
$5.00/5G

444731-73-1
209 suppliers
$25.00/100mg
Yield:444731-73-1 97%
Reaction Conditions:
at 80; for 0.025 h;Flow reactor;Temperature;
Steps:
1.3; 2.3; 3.3; 1.3
(3) take by weighing the PZPN-2A of 100g, add stirring and dissolving in the dimethyl carbonate of 200g to form material 5, take by weighing the dimethyl carbonate of 200g and form material 6, adjust the flow velocity of slurry pump B to make the flow velocity of material 5 be 10ml/min, adjust the flow rate of flow pump D to make the flow rate of material 2 be 5ml/min, the reaction temperature is 80 , the mass ratio of PZPN-2A and dimethyl carbonate is 1: 2.0, and the reaction residence time is 90s, collected from The reaction solution flowing out of the outlet, after the reaction solution was added to water, precipitated a solid, and filtered to obtain PZPN-3A with a yield of 97% and a purity of 99%.
References:
CN114380748,2022,A Location in patent:Paragraph 0015; 0050; 0053; 0055; 0058; 0060; 0063; ...

6494-19-5
260 suppliers
$19.00/1g

616-38-6
722 suppliers
$5.00/5G

444731-73-1
209 suppliers
$25.00/100mg

6494-19-5
260 suppliers
$19.00/1g
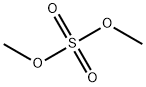
77-78-1
319 suppliers
$22.00/25g

444731-73-1
209 suppliers
$25.00/100mg

6494-19-5
260 suppliers
$19.00/1g
74-88-4
357 suppliers
$15.00/10g

444731-73-1
209 suppliers
$25.00/100mg

6494-19-5
260 suppliers
$19.00/1g
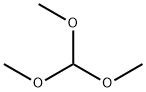
149-73-5
550 suppliers
$12.00/5g

444731-73-1
209 suppliers
$25.00/100mg