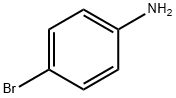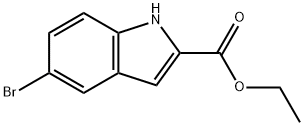
Ethyl 5-Bromoindole-2-carboxylate synthesis
- Product Name:Ethyl 5-Bromoindole-2-carboxylate
- CAS Number:16732-70-0
- Molecular formula:C11H10BrNO2
- Molecular Weight:268.11

7254-19-5
363 suppliers
$9.00/250mg

64-17-5
825 suppliers
$10.00/50g

16732-70-0
331 suppliers
$6.00/1g
Yield:16732-70-0 94%
Reaction Conditions:
with sulfuric acid at 80; for 12 h;
Steps:
20.a a) 5-Bromo-lH-indole-2-carboxylic acid ethyl ester
Example 20 5-(l-Isopropyl-piperidin-4-yl)-lH-indole-2-carboxylic acid (3-fluoro-phenyl)-amide a) 5-Bromo-lH-indole-2-carboxylic acid ethyl ester 5-Bromoindole-2-carboxylic acid (2.5 g; 10.4 mmol) was dissolved in ethanol (100 mL). Concentrated sulphuric acid (2.5 mL) was added and the reaction mixture heated at 80°C for 12 hours. The mixture was cooled to room temperature and the solution neutralized to pH 7 using aqueous sodium bicarbonate solution (50 mL). The reaction mixture was concentrated in vacuo to remove ethanol, and the aqueous phase was extracted using ethyl acetate (2 x 100 mL). The combined organic phases were dried over magnesium sulfate, filtered and concentrated in vacuo to afford the title compound (2.63 g, 94 %). HPLC-MS (purity; retention time) = 100 %; 2.31 min. MS ISP (m/e): 270.0/268.1 (100/100) [(M+H)+]
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;BAUMANN, Karlheinz;FLOHR, Alexander;JOLIDON, Synese;KNUST, Henner;LUEBBERS, Thomas;NETTEKOVEN, Matthias WO2014/60386, 2014, A1 Location in patent:Page/Page column 43
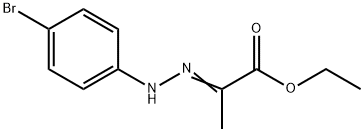
16382-11-9
24 suppliers
inquiry

16732-70-0
331 suppliers
$6.00/1g

617-35-6
564 suppliers
$5.00/10g

622-88-8
314 suppliers
$8.00/5g

16732-70-0
331 suppliers
$6.00/1g

16382-11-9
24 suppliers
inquiry