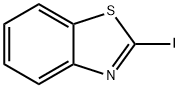
2-IODOBENZOTHIAZOLE synthesis
- Product Name:2-IODOBENZOTHIAZOLE
- CAS Number:1123-99-5
- Molecular formula:C7H4INS
- Molecular Weight:261.08

95-16-9

1123-99-5
The general procedure for the synthesis of 2-iodobenzothiazole from benzothiazole was as follows: benzothiazole (1 mmol, 135.9 mg) and perfluorobutyl iodide (1.1 mmol, 380.5 mg) were placed in a 10 mL round bottom flask. 5 mL of N,N-dimethylformamide and sodium tert-butoxide (0.5 mmol, 48.1 mg) were added. The reaction mixture was stirred at room temperature for 20 min and the progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was poured into water and extracted with dichloromethane. The organic phase was collected and dried with anhydrous sodium sulfate, followed by removal of dichloromethane by rotary evaporator to give the crude product. The crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate (v/v=30:1) as eluents to give 2-iodobenzothiazole (white solid, 257.6 mg, 99% yield). In addition, the crude product can also be purified by recrystallization: the crude product was first washed with cold water, filtered, and dissolved in ethanol (2 mL). After heating until complete dissolution, it was left to stand and slowly cooled to induce precipitation of crystals.

136-95-8
417 suppliers
$7.00/25g

1123-99-5
111 suppliers
$19.00/250mg
Yield: 84.7%
Reaction Conditions:
with toluene-4-sulfonic acid;potassium iodide;sodium nitrite in water;acetonitrile at 0 - 20;
Steps:
2-iodobenzo[d]thiazole (7)
To a solution of 2-aminobenzothiazole (6) (3.75g, 25mol) and p-TsOH (15g, 75mmol) in acetonitrile (100ml) was added a mixture solution of KI (10.5g, 65mmol) and NaNO2 (3.5g, 50mol) in water (15ml) at 0°C. The reaction solution was stirred at 0°C for 2h, and then at RTovernight. To the reaction solution was added around 350ml of water, adjust the solution pH to 8-9 with NaHCO3 and added Na2S2O3, filtered and the solid was washed to give brown solid 5.52g (yield 84.7%). TLC: Rf: 0.6 (hexane/ethyl acetate=6/1). MS (ESI) m/z 262 (M++H);1HNMR (CDCl3, 300MHz) δ 8.06 (1H, dd, J1=7.5Hz, J2=1.8Hz), 7.87 (1H, dd, J1=6.9Hz,J2=1.5Hz), 7.50~7.38 (2H, m).
References:
Qi, Jianjun;Tung, Ching-Hsuan [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 1,p. 320 - 323] Location in patent:supporting information; experimental part

95-16-9
426 suppliers
$14.14/10gm:

1123-99-5
111 suppliers
$19.00/250mg
615-20-3
250 suppliers
$19.00/25g

1123-99-5
111 suppliers
$19.00/250mg

95-16-9
426 suppliers
$14.14/10gm:

6574-98-7
340 suppliers
$6.00/5g

1123-99-5
111 suppliers
$19.00/250mg

1402247-75-9
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1123-99-5
111 suppliers
$19.00/250mg