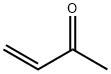
3-(Hydroxymethyl)-3-butene-2-one synthesis
- Product Name:3-(Hydroxymethyl)-3-butene-2-one
- CAS Number:73255-29-5
- Molecular formula:C5H8O2
- Molecular Weight:100.12
Yield:73255-29-5 201 mg
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane in ethanol at 0 - 20; for 3 h;Sealed tube;Morita-Baylis-Hillman Alkylation;
Steps:
3-(Hydroxymethyl)but-3-en-2-one (4a)
According to general procedure B - neat mode, paraformaldehyde (100 mg, 3.32 mmol, 1.4 equiv.) was cracked in ethanol (0.40 mL, 6.85 mmol,2.9 equiv.) using DABCO (13 mg, 0.12 mmol, 0.05 equiv.). After cooling,methyl vinyl ketone (3a, 166 mg, 2.37 mmol, 1 equiv.) was added and thereaction was stirred for 3 h at room temperature. Subsequently, purification was conducted bycolumn chromatography (50% diethyl ether in n-pentane). Due to the volatility of thecompound, the solvent was carefully removed under reduced pressure (rotational evaporator,35 °C, minimum pressure 960 mbar. Then slowly lower pressure, until no more solvent left), toobtain the product as a colorless liquid (201 mg, 2.01 mmol, 85%).RF = 0.29 (50% ethyl acetate in petroleum ether); IR (atr film) [cm-1] = 3413, 2997, 2927,2871, 1735, 1664, 1630, 1429, 1368, 1317, 1300, 1231, 1130, 1047, 1027, 969, 946, 779, 726,706, 668; 1H-NMR (600 MHz, CDCl3): δH [ppm] = 2.34 (s, 3H, 4-H), 2.64 (s, 1H, OH), 4.29 (s,2H, H-1), 6.03 (s, 1H, 2’-Ha), 6.11 (s, 1H, 2’-Hb).; 13C-NMR (151 MHz, CDCl3): δC [ppm] = 26.0(C-4), 62.3 (C-1), 126.3 (C-2’), 147.3 (C2), 200.5 (C3); MS (EI, positive Ion, 70 eV): m/z (%) =100 (>5) [M+], 85 (100) [M-Me], 57 (9) [C3H5O+]; HRMS (C5H9O2): calc. 101.0603, found 101.0597.
References:
Mantel, Marvin;Guder, Marian;Pietruszka, J?rg [Tetrahedron,2018,vol. 74,# 38,p. 5442 - 5450] Location in patent:supporting information