
3-TERT-BUTYL-1-(2-METHYLPHENYL)-1H-PYRAZOL-5-AMINE synthesis
- Product Name:3-TERT-BUTYL-1-(2-METHYLPHENYL)-1H-PYRAZOL-5-AMINE
- CAS Number:337533-96-7
- Molecular formula:C14H19N3
- Molecular Weight:229.32

59997-51-2
270 suppliers
$6.00/25g

635-26-7
200 suppliers
$5.00/1g

337533-96-7
33 suppliers
$45.00/50mg
Yield:337533-96-7 93%
Reaction Conditions:
Stage #1: trimethylacetylacetonitrile;o-tolylhydrazine hydrochloridewith acetic acid in ethanol; for 18 h;Heating / reflux;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
F
4,4-Dimethyl-3-oxopentanenitrile (36.7 g, 0.29 mol), (2-methylphenyl)hydrazine hydrochloride (47.7 g, 0.29 mol), and glacial acetic acid (7.03 g, 6.7 mL, 0.12 mol) were dissolved in abs ethanol (585 mL) and heated under reflux for 18 h. After removal of the solvent under reduced pressure, EtOAc and water (500 mL each) were added, then sodium bicarbonate (42 g, 0.50 mol) was carefully added. After addition of hexane (500 mL), the organic phase was separated, washed with brine (500 mL), and dried over Na2SO4. The mixture was then filtered through a pad of silica gel (500 g) on a sintered glass funnel. The pad was eluted with hexanes/EtOAc (1:1, v/v), and the filtrate was concentrated under reduced pressure. The resulting solid was triturated with hexanes/EtOAc (9:1, v/v), filtered, washed and dried in vacuo to afford the product as a colorless solid (61.5 g, 93%). 1H NMR (400 MHz, CD2Cl2) 61.29 (s, 9H), 2.12 (s, 3H), 3.56 (br, 2H), 5.48 (s, 1H), 7.28 (m, 2H), 7.31 (m, 2H).
References:
US2005/192294,2005,A1 Location in patent:Page/Page column 13

59997-51-2
270 suppliers
$6.00/25g
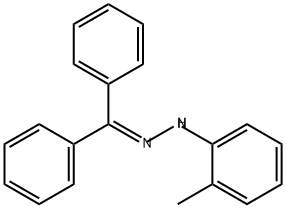
213257-69-3
0 suppliers
inquiry

337533-96-7
33 suppliers
$45.00/50mg

59997-51-2
270 suppliers
$6.00/25g

529-27-1
15 suppliers
inquiry

337533-96-7
33 suppliers
$45.00/50mg