
4-Bromo-7-azaindole synthesis
- Product Name:4-Bromo-7-azaindole
- CAS Number:348640-06-2
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03

55052-24-9

348640-06-2
1H-pyrrolo[2,3-b]pyridine-7-oxide (3.0 g, 22.4 mmol) was used as starting material, which was co-dissolved with tetramethyl ammonium (4.13 g, 1.2 eq.) in N,N-dimethylformamide (DMF, 30 ml). Dimethyl sulfoxide (Ms2O, 7.8 g, 2.0 eq.) was added in batches at 0 °C. The reaction mixture was stirred at 0 °C for 1 h, then brought to room temperature and continued stirring for 4 h. The reaction was carried out at 0 °C. Upon completion of the reaction, the reaction solution was diluted with water (60 ml) and the pH was adjusted with solid sodium hydroxide (NaOH) to 7. Subsequently, more than 130 ml of water was added and the resultant suspension was kept at 5 °C for 1 h. The reaction was carried out by filtration. The precipitate was collected by filtration, washed with ice water (2 x 20 ml) and dried in a vacuum oven using phosphorus pentoxide (P2O5) as a desiccant to give finally 4-bromo-1H-pyrrolo[2,3-b]pyridine (2.47 g, 56% yield). Mass spectrum (ESI) m/z 196.9 [M + H]+. 1H NMR (400 MHz, CDCl3) δ 10.77 (br s, 1H), 8.14 (d, J = 5.2 Hz, 1H), 7.42 (s, 1H), 7.31 (d, J = 5.1 Hz, 1H), 6.57 (s, 1H).

55052-24-9
145 suppliers
$80.00/5 g

348640-06-2
339 suppliers
$6.00/250mg
Yield:348640-06-2 56%
Reaction Conditions:
with tetramethylammonium bromide;Methanesulfonic anhydride in N,N-dimethyl-formamide at 0 - 20; for 5 h;
Steps:
1H-pyrrolo[2,3-b]pridine 7-oxide 21 (3.0 g, 22.4 mmol) and tetramethylammonium (4.13 g, 1.2 eq) were dissolved in DMF (30 ml). The Ms2O (7.8 g, 2.0 eq) was added at 0 in small portions. The obtained mixture was stirred for 1 h at 0 and for 4 h at room temperature and diluted with water (60 ml). The PH was adjusted to 7 with solid NaOH, and more than 130 ml of water was added. The resulting suspension was kept at 5 for 1 h. The precipitate was separated by filtration, washed with ice-water (2 * 20 ml), and dried over P2O5 in vacuum oven to give 4-bromo-1H-pyrrolo[2,3-b]pyridine (2.47 g, 56%). MS (ESI) m/z 196.9 [M+H]+. 1H NMR (400 Hz, CDCl3) δ 10.77 (br, s, 1H), 8.14 (d, J = 5.2 Hz1H), 7.42 (s, 1H), 7.31 (d, J = 5.1 Hz1H), 6.57 (s, 1H).
References:
Chang, Shaohua;Zhang, Zhang;Zhuang, Xiaoxi;Luo, Jinfeng;Cao, Xianwen;Li, Honglin;Tu, Zhengchao;Lu, Xiaoyun;Ren, Xiaomei;Ding, Ke [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 2,p. 1208 - 1212] Location in patent:supporting information; experimental part

55052-24-9
145 suppliers
$80.00/5 g
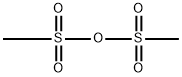
7143-01-3
276 suppliers
$19.00/5g

348640-06-2
339 suppliers
$6.00/250mg

55052-24-9
145 suppliers
$80.00/5 g

1000340-33-9
62 suppliers
$94.00/100mg

348640-06-2
339 suppliers
$6.00/250mg

271-63-6
579 suppliers
$9.00/5g

348640-06-2
339 suppliers
$6.00/250mg