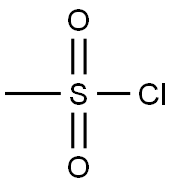4-Chloro-7-azaindole synthesis
- Product Name:4-Chloro-7-azaindole
- CAS Number:55052-28-3
- Molecular formula:C7H5ClN2
- Molecular Weight:152.58

271-63-6
573 suppliers
$9.00/5g

55052-28-3
477 suppliers
$6.00/1g
Yield:55052-28-3 85%
Reaction Conditions:
with 3-chloro-benzenecarboperoxoic acid;trichlorophosphate in 1,2-dimethoxyethane;n-heptane at 20 - 85; for 18 h;
Steps:
1 Preparation of 4-chloro-1-(triisopropylsilyl)-lH-pyrrolo [2,3-b]pyridine (1)
7-azaindole (3.6 g, 30 mmol) was dissolved in dimethoxyethane (17 ml) and heptane (33 ml), meta-chloroperoxybenzoic acid (mCPBA) (8.1 g, 77% And the mixture is stirred at room temperature. The mixture was filtered through a filter paper using a solution of dimethoxyethane (34 ml) and heptane (64 ml), phosphorus oxychloride (POCl3) (22 ml, 0.24 mol) was added to the obtained compound,For 18 hours The mixture is heated under reflux at 80°C . The mixture is sufficiently cooled, diluted with water (150 ml), and adjusted to pH 10 with 6N sodium hydroxide (NaOH). The mixture was filtered on a filter paper using water to obtain a solid compound (1) having a pale orange color. (3.9 g, 85% yield):
References:
KR101548492,2015,B1 Location in patent:Paragraph 0048; 0049
![Benzoic acid, 3-chloro-, compd. with 7-hydroxy-7H-pyrrolo[2,3-b]pyridine (1:1)](/CAS/20211123/GIF/74420-18-1.gif)
74420-18-1
0 suppliers
inquiry

55052-28-3
477 suppliers
$6.00/1g

55052-24-9
144 suppliers
$80.00/5G

55052-28-3
477 suppliers
$6.00/1g
![1H-Pyrrolo[2,3-b]pyridine, 4-chloro-1-(phenylsulfonyl)-](/CAS/GIF/744209-63-0.gif)
744209-63-0
85 suppliers
$45.00/10mg

55052-28-3
477 suppliers
$6.00/1g