
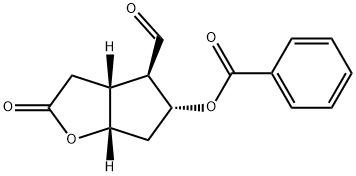
(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one synthesis
- Product Name:(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one
- CAS Number:55076-60-3
- Molecular formula:C25H24O5
- Molecular Weight:404.46

41162-19-0
183 suppliers
inquiry
39746-01-5
77 suppliers
$140.00/500mg
![(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one](/CAS2/GIF/55076-60-3.gif)
55076-60-3
62 suppliers
$59.00/2 mg
Yield: 84.7%
Reaction Conditions:
Stage #1:dimethyl(2-oxo-4-phenylbutyl)phosphonate with N-ethyl-N,N-diisopropylamine;lithium chloride in acetonitrile at -15;
Stage #2:(1S,5R,6R,7R)-6-formyl-7-benzoyloxy-2-oxabicyclo[3.3.0]octan-3-one in acetonitrile at -15;Wittig Reaction;
Steps:
2
To a suspension of 21g of LiCl (MW = 42.39; 4.4 eq.) in 280mL of acetonitrile was added 37g of compound 19a (MW = 256.25; 1.3 eq.; prepared as described in example 1). The resulting mixture is cooled to -15°C and 45mL of Hünig's base (MW = 129.25; d =0.755; 3.0 eq.) were added. After stirring for 1h at -15°C 520mL of the benzoyl protected Corey aldehyde solution obtained as described above were added. After stirring for 1 h 19mL of glacial acetic acid were added before the reaction mixture was warmed to ambient temperature. The reaction mixture was washed consecutively with an aqueous NaCl solution (18%) and water. Then the main part of the organic solvents were removed by distillation under reduced pressure and pre-warmed (80°C) ethanol (96%) was added. Then the mixture was distilled under reduced pressure until the desired product started to crystallize from the mixture. The crystals were collected by filtration after cooling the suspension to 0°C and washed twice an ice-cold mixture of ethanol / water (1 / 1) to yield 38g of the title compound after drying (40°C, 10mbar; yield = 84.7%; assay: 99.2%). [Show Image] 1H-NMR (CDCl3, 300MHz) δ(ppm) = 2.35 (dd, CH2, 1 H, J 10.6Hz, J 3.1 Hz), 2.60 (m, CH2, 3H), 2.90 (m, CH, CH2, 5H), 5.09 (dt, CH, 1 H, J 4.3Hz, 1.8Hz), 5.33 (q, CH, 1H, J 5.5Hz), 6.25 (d, CH, 1H, 16.4Hz), 6.70 (m, CH, 1H), 7.25 (m, CH, 5H), 7.49 (m, CH, 2H), 7.61 (m, CH, 1 H), 8.02(m, CH, 2H). 13C-NMR (CDCl3, 75.47MHz) δ(ppm) = 30.37, 35.28, 38.23, 42.87, 42.95, 54.43, 78.89, 83.54, 126.64, 128.81, 128.95, 129.01, 129.64, 130.11, 131.86, 133.96, 141.29, 143.48, 166.28, 176.26, 199.06.
References:
SANDOZ AG EP2143712, 2010, A1 Location in patent:Page/Page column 53-54
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

39746-01-5
77 suppliers
$140.00/500mg
![(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one](/CAS2/GIF/55076-60-3.gif)
55076-60-3
62 suppliers
$59.00/2 mg

40601-45-4
6 suppliers
inquiry

39746-01-5
77 suppliers
$140.00/500mg
![(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one](/CAS2/GIF/55076-60-3.gif)
55076-60-3
62 suppliers
$59.00/2 mg
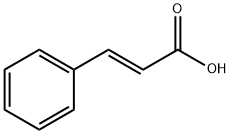
140-10-3
643 suppliers
$5.00/10g
![(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one](/CAS2/GIF/55076-60-3.gif)
55076-60-3
62 suppliers
$59.00/2 mg

103-25-3
263 suppliers
$9.00/1g
![(3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-5-phenyl-1-pentenyl]-2H-cyclopenta[b]furan-2-one](/CAS2/GIF/55076-60-3.gif)
55076-60-3
62 suppliers
$59.00/2 mg