
Atovaquone synthesis
- Product Name:Atovaquone
- CAS Number:95233-18-4
- Molecular formula:C22H19ClO3
- Molecular Weight:366.84

137732-39-9
48 suppliers
$145.00/1mg

95233-18-4
401 suppliers
$19.00/5mg
Yield:95233-18-4 85%
Reaction Conditions:
with sulfuric acid at 15 - 16; for 0.333333 h;
Steps:
1
Cis-2-[4-(4-chlorophenyl)cyclohexyl]-3 -hydroxy- 1,4-naphthoquinone (1 g, 2.7 mmol) was stirred in 8 ml concentrated H2SO4 at 15-16°C for 20 minutes. The reaction mixture was slowly poured into 30 g ice and further stirred for 20 minutes. The obtained solid was filtered and washed with water until the pH of the filtrate becomes in the range of 4 to 5. The resulting solid is dried at 50-55°C for 6 hours to yield 0.85 g of trans-2-[4-(4- chlorophenyl)cyclohexyl]-3-hydroxy-l,4-naphthoquinone (85% yield, 95.5 % purity).
References:
CHEMAGIS LTD.;ZHU, Fuqiang;BEKHAZI, Michel WO2010/1378, 2010, A1 Location in patent:Page/Page column 11

94015-53-9
4 suppliers
inquiry

95233-18-4
401 suppliers
$19.00/5mg
![1,4-Naphthalenedione, 2-chloro-3-[4-(4-chlorophenyl)cyclohexyl]-](/CAS/20210111/GIF/129719-64-8.gif)
129719-64-8
0 suppliers
inquiry

95233-18-4
401 suppliers
$19.00/5mg

94015-53-9
4 suppliers
inquiry

137732-39-9
48 suppliers
$145.00/1mg

95233-18-4
401 suppliers
$19.00/5mg
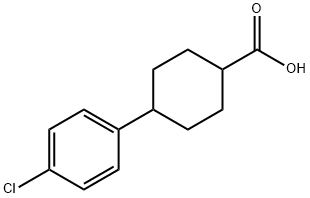
95233-37-7
135 suppliers
$8.00/5g

95233-18-4
401 suppliers
$19.00/5mg