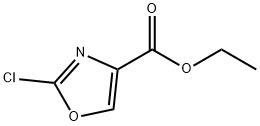
ETHYL 2-CHLOROOXAZOLE-4-CARBOXYLATE synthesis
- Product Name:ETHYL 2-CHLOROOXAZOLE-4-CARBOXYLATE
- CAS Number:460081-18-9
- Molecular formula:C6H6ClNO3
- Molecular Weight:175.57

177760-52-0

460081-18-9
Under nitrogen protection, 1.70 mL (14.3 mmol) of tert-butyl nitrite was added dropwise to a suspension of 1.65 g (12.3 mmol) cuprous(I) chloride in 50 mL of acetonitrile. The reaction mixture was heated to 75 °C, followed by the addition of 1.60 g (10.2 mmol) of ethyl 2-aminooxazole-4-carboxylate in batches over 20 min (Ref: G Crank MJ Foulis, J Med Chem 1971,14:1075-1077). Gases were released during the reaction. After continued stirring for 30 min, the reaction mixture was cooled to room temperature, diluted with 50 mL of ethyl acetate and washed with water (2 x 25 mL). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure to give a dark oily solid. Purification by neutral silica gel column chromatography using a solvent mixture of hexane and ethyl acetate (3:1) afforded 1.27 g (71% yield) of the target compound, ethyl 2-chloroxazole-4-carboxylate, as white needle-like crystals (recrystallized from hexane). Mass spectrum (electrospray positive ion mode): m/z 176/177 [M+H]+. 1H NMR (CDCl3) δ: 1.47 (t, 3H, J = 7.16 Hz); 4.48 (q, 2H, J = 7.16 Hz); 8.28 (s, 1H).

70-23-5
317 suppliers
$6.00/5g

460081-18-9
167 suppliers
$10.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 100 °C
2: 83 percent / t-BuONO; CuCl2 / acetonitrile / 2 h / 80 °C
References:
Hodgetts, Kevin J.;Kershaw, Mark T. [Organic Letters,2002,vol. 4,# 17,p. 2905 - 2907]

177760-52-0
211 suppliers
$6.00/250mg

460081-18-9
167 suppliers
$10.00/250mg

540-80-7
386 suppliers
$29.00/25 g

177760-52-0
211 suppliers
$6.00/250mg

7447-39-4
264 suppliers
$10.00/25g

460081-18-9
167 suppliers
$10.00/250mg