
ETHYL (S)-3-HYDROXYHEXANOATE synthesis
- Product Name:ETHYL (S)-3-HYDROXYHEXANOATE
- CAS Number:88496-71-3
- Molecular formula:C8H16O3
- Molecular Weight:160.21

3249-68-1
253 suppliers
$6.00/5g

88496-71-3
19 suppliers
inquiry
Yield:88496-71-3 100%
Reaction Conditions:
Stage #1: with triethylamine;dichloro(1,5-cyclooctadiene)ruthenium(II);(S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine) in toluene at 140; for 4 h;
Stage #2: ethyl butyroyl acetatewith hydrogen in methanol at 80; under 3345.86 Torr; for 5 h;
Steps:
4
Catalyst (2): Combine dichloro (1, 5-cyclooctadiene) ruthenium (117 mg, 0.417 mmol), (59-BINAP (300 mg, 0.482 mmol), and NEt3 (82. 5 pL, 0.59 mmol) in anhydrous toluene (13.5 mL) under N2. Heat the reaction mixture at 140° for 4 hours, and then cool to ambient temperature. Add THF to the resulting red jell, until a solution results. Concentrate the reaction mixture in vacuo, and add THF (30 mL) to the give the catalyst (1) in solution. This solution is used directly for the hydrogenation. (S)-3-Hydroxy-hexanoic acid ethyl ester: In a Fisher Porter tube, add the above catalyst (2) solution (10 mL) to a solution of ethyl butyrylacetate (25 g, 0.158 mol) in methanol (100 mL) under N2. Pressurize with H2 (50 psig) and heat at 80°C for 5 hours. Concentrate the reaction mixture ira vacuo. Purify the residue by vacuum distillation (25.0 g, 100%, 97.4 % ee). Chiral GC (30 m x 0.25 mm x 0. 25/im, BETA- Dex 225, 100°C) first eluting isomer, (18.72 minutes).
References:
WO2005/92835,2005,A1 Location in patent:Page/Page column 21

29117-54-2
9 suppliers
inquiry

88496-71-3
19 suppliers
inquiry
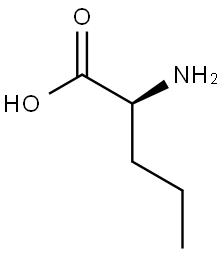
6600-40-4
570 suppliers
$5.00/1g

88496-71-3
19 suppliers
inquiry

41014-93-1
62 suppliers
$22.00/100mg

88496-71-3
19 suppliers
inquiry
![Hexanoic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, ethyl ester, (3S)-](/CAS/20210305/GIF/123807-97-6.gif)
123807-97-6
0 suppliers
inquiry

88496-71-3
19 suppliers
inquiry