4-(Boc-Aminomethyl)piperidine
- CAS No.
- 135632-53-0
- Chemical Name:
- 4-(Boc-Aminomethyl)piperidine
- Synonyms
- TERT-BUTYL (PIPERIDIN-4-YLMETHYL)CARBAMATE;tert-Butyl N-(piperidin-4-ylmethyl)carbamate;PIP(4-BOC-AM);BUTTPARK 43\57-92;(BOC-4-AMINOMETHYL)PIPERIDINE;4-(BOC-AMINOMETHYL)PIPERIDINE;4-N-BOC-AMINOMETHYL PIPERIDINE;4-(2-BOC-AMINOMETHYL) PIPERIDINE;me: 4-(Boc-Aminomethyl)piperidine;4-(Boc-aminomethyl)piperidine,97%
- CBNumber:
- CB4102632
- Molecular Formula:
- C11H22N2O2
- Molecular Weight:
- 214.3
- MDL Number:
- MFCD01631214
- MOL File:
- 135632-53-0.mol
| Product description | Number | Pack Size | Price |
| 4-(tert-Butoxycarbonylaminomethyl)piperidine >98.0%(GC)(T) | B4444 | 1g | $344 |
| tert-Butyl(Piperidin-4-ylmethyl)carbamate | B701450 | 1g | $75 |
| tert-Butyl (piperidin-4-ylmethyl)carbamate 95+% | 016373 | 5g | $48 |
| 4-(N-Boc-aminomethyl)piperidine | FB36900 | 2g | $50 |
| 4-(Boc-aminomethyl)piperidine 97% | JK989088 | 1g | $67 |
| More product size | |||
| Melting point | 106 °C |
|---|---|
| Boiling point | 321.8±15.0 °C(Predicted) |
| Density | 0.981±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO. |
| pka | 12.72±0.46(Predicted) |
| form | powder to crystal |
| color | White to Almost white |
| CAS DataBase Reference | 135632-53-0(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H318 | |||||||||
| Precautionary statements | P260h-P301+P330+P331-P303+P361+P353-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 34-36/38 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | 3259 | |||||||||
| Hazard Note | Corrosive | |||||||||
| HazardClass | IRRITANT | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | Ⅲ | |||||||||
| HS Code | 29333990 | |||||||||
| NFPA 704 |
|
4-(Boc-Aminomethyl)piperidine price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | B4444 | 4-(tert-Butoxycarbonylaminomethyl)piperidine >98.0%(GC)(T) | 135632-53-0 | 1g | $344 | 2025-07-31 | Buy |
| TRC | B701450 | tert-Butyl(Piperidin-4-ylmethyl)carbamate | 135632-53-0 | 1g | $75 | 2021-12-16 | Buy |
| Matrix Scientific | 016373 | tert-Butyl (piperidin-4-ylmethyl)carbamate 95+% | 135632-53-0 | 5g | $48 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB36900 | 4-(N-Boc-aminomethyl)piperidine | 135632-53-0 | 2g | $50 | 2021-12-16 | Buy |
| Frontier Specialty Chemicals | JK989088 | 4-(Boc-aminomethyl)piperidine 97% | 135632-53-0 | 1g | $67 | 2021-12-16 | Buy |
4-(Boc-Aminomethyl)piperidine Chemical Properties,Uses,Production
Uses
4-(Boc-aminomethyl)piperidine is used as an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff.
Synthesis

7144-05-0
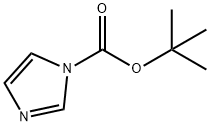
49761-82-2

135632-53-0
General procedure: 4-aminomethylpiperidine (3.6 g) and 1-BOC-imidazole (5.3 g) were dissolved in toluene (80 mL), and the reaction was stirred at 25°C overnight. After completion of the reaction, the solution was concentrated and the residue was purified by silica gel column chromatography (eluent: EtOAc/hexane=1/2) to afford Intermediate 227-I (4.7 g) in 70% yield. Intermediate 227-I (4.7 g) and triethylamine (2.7 mL) were dissolved in 1-pentanol (20 mL), 2,4-dichloro-6-aminopyrimidine (5.4 g) was added, and the reaction was carried out at 120 °C for 12 hours. After completion of the reaction, the solvent was removed and the residue was purified by silica gel column chromatography (eluent: EtOAc/hexane=1/9) to afford Intermediate 227-II (5.2 g) in 70% yield. Intermediate 227-II (1.0 g) was treated with 1 M HCl (20 mL) in CH2Cl2 (10 mL) and stirred at room temperature for 8 hours. After completion of the reaction, the solution was concentrated, the residue neutralized with NH4OH and extracted with CH2Cl2. The organic layer was separated and concentrated, and the residue was purified by silica gel column chromatography (eluent: MeOH) to afford Intermediate 227-III (636 mg) in 90% yield. Intermediate 222-III (790 mg) was added to a solution of intermediate 227-III (450 mg) in MeOH (20 mL) and stirred at 25 °C for 2 hours. Then NaBH(OAc)3 (2.0 g) was added and the reaction was continued at 25 °C for 12 hours. After completion of the reaction, the solution was concentrated, saturated NaHCO3 solution was added and extracted with CH2Cl2. The organic layer was separated and concentrated, and the residue was purified by silica gel column chromatography (eluent: MeOH) to afford intermediate 227-IV (539 mg) in 60% yield. N1-morpholino-N1-piperazine ethane (240 mg) was added to a 1-pentanol (1 mL) solution of Intermediate 227-IV (160 mg) and stirred at 120 °C for 8 hours. After completion of the reaction, the solution was concentrated and the residue was purified by silica gel column chromatography (eluent: EtOAc/MeOH=5/1) to afford Intermediate 227-V (85 mg) in 40% yield. 20% TFA/CH2Cl2 (1 mL) was added to a solution of CH2Cl2 (1 mL) of intermediate 227-V (85 mg) and stirred at room temperature for 8 hours. After completion of the reaction, the solvent was removed and the residue was purified by silica gel column chromatography (eluent: 21% NH3(aq)/MeOH=1/19) to afford compound 227 (65 mg) in 90% yield. Finally, compound 227 was treated with a solution of CH2Cl2 (1 mL) in 1M HCl (1 mL) for 0.5 hours. After removal of the solvent, the residue was treated with ether and filtered to give the hydrochloride salt of compound 227.CI-MS (M++1): 544.4.
References
[1] Patent: US2006/281712, 2006, A1. Location in patent: Page/Page column 108-109
4-(Boc-Aminomethyl)piperidine Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | +86-(0)57185586718; +8613336195806 | sales@capot.com | China | 29734 | 60 |
| Chembon Pharmaceutical Co., Ltd. | +86-28-8425-2981 | CHINA | 722 | 55 | |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3009 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29852 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Accela ChemBio Inc. | +1-858-6993322 | info@accelachem.com | United States | 21193 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5906 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49975 | 58 |
View Lastest Price from 4-(Boc-Aminomethyl)piperidine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-04 | 4-(Boc-Aminomethyl)piperidine
135632-53-0
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2022-01-26 | 4-(Boc-Aminomethyl)piperidine
135632-53-0
|
US $0.00 / KG | 1KG | 97.8% | 100 tons | Honest Joy Holdings Limited | |
 |
2019-09-02 | me: 4-(Boc-Aminomethyl)piperidine
135632-53-0
|
US $1.00 / KG | 1KG | 98% | 1kg; 2kg;10kg; 100kg | Career Henan Chemical Co |
-

- 4-(Boc-Aminomethyl)piperidine
135632-53-0
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- 4-(Boc-Aminomethyl)piperidine
135632-53-0
- US $0.00 / KG
- 97.8%
- Honest Joy Holdings Limited
-

- me: 4-(Boc-Aminomethyl)piperidine
135632-53-0
- US $1.00 / KG
- 98%
- Career Henan Chemical Co






