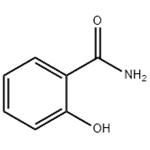
Salicylamide
- CAS No.
- 65-45-2
- Chemical Name:
- Salicylamide
- Synonyms
- 2-HYDROXYBENZAMIDE;Cetamide;Salamide;Algamon;Benesal;Salicylamid;OHB;Acket;Samid;Cidal
- CBNumber:
- CB4854078
- Molecular Formula:
- C7H7NO2
- Molecular Weight:
- 137.14
- MDL Number:
- MFCD00007978
- MOL File:
- 65-45-2.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| Salicylamide puriss., ≥99.0% (T) | 84230 | 1kg | $76.3 |
| Salicylamide United States Pharmacopeia (USP) Reference Standard | 1608000 | 200mg | $376 |
| Salicylamide >98.0%(T) | S0006 | 25g | $16 |
| Salicylamide >98.0%(T) | S0006 | 500g | $56 |
| Salicylamide Pharmaceutical Secondary Standard; Certified Reference Material | PHR1449 | 1g | $107.35 |
| More product size | |||
| Melting point | 140-144 °C(lit.) |
|---|---|
| Boiling point | 270°C |
| Density | 1,175 g/cm3 |
| refractive index | 1.5323 (estimate) |
| Flash point | 181°C/14mm |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | methanol: 0.1 g/mL, clear |
| pka | pKa 8.13(H2O t = 37) (Uncertain) |
| form | Crystalline Powder |
| color | White |
| Odor | Odorless |
| PH Range | 5 (0.2% aq soln) |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Decomposition | 270°C |
| Merck | 14,8328 |
| BRN | 742439 |
| Stability | Stable. Light sensitive. Incompatible with strong bases, strong oxidizing agents. |
| Cosmetics Ingredients Functions | KERATOLYTIC |
| InChI | 1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10) |
| InChIKey | SKZKKFZAGNVIMN-UHFFFAOYSA-N |
| SMILES | NC(=O)c1ccccc1O |
| LogP | 1.280 |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 65-45-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | EM8BM710ZC |
| ATC code | N02BA05 |
| NIST Chemistry Reference | Benzamide, 2-hydroxy-(65-45-2) |
| EPA Substance Registry System | o-Hydroxybenzamide (65-45-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P270-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| target organs | Respiratory system | |||||||||
| PPE | dust mask type N95 (US), Eyeshields, Gloves | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | VN6475000 | |||||||||
| TSCA | TSCA listed | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29214300 | |||||||||
| Storage Class | 11 - Combustible Solids | |||||||||
| Hazard Classifications | Acute Tox. 4 Oral Eye Irrit. 2 Skin Irrit. 2 STOT SE 3 |
|||||||||
| Hazardous Substances Data | 65-45-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in mice: 1.4 g/kg (Hart) | |||||||||
| NFPA 704 |
|
Salicylamide price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 84230 | Salicylamide puriss., ≥99.0% (T) | 65-45-2 | 1kg | $76.3 | 2025-07-31 | Buy |
| Sigma-Aldrich | 1608000 | Salicylamide United States Pharmacopeia (USP) Reference Standard | 65-45-2 | 200mg | $376 | 2025-07-31 | Buy |
| TCI Chemical | S0006 | Salicylamide >98.0%(T) | 65-45-2 | 25g | $16 | 2025-07-31 | Buy |
| TCI Chemical | S0006 | Salicylamide >98.0%(T) | 65-45-2 | 500g | $56 | 2025-07-31 | Buy |
| Sigma-Aldrich | PHR1449 | Salicylamide Pharmaceutical Secondary Standard; Certified Reference Material | 65-45-2 | 1g | $107.35 | 2025-07-31 | Buy |
Salicylamide Chemical Properties,Uses,Production
Description
Salicylamide is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable solutions can be formed at pH 9 through ionization of the phenolic group. It is absorbed from the GI tract on oral administration and is rapidly metabolized to inactive metabolites by intestinal mucosa, but not by hydrolysis. Activity appears to reside in the intact molecule. It is approximately 40 to 55% plasma protein bound, and it competes with other salicylates and acetaminophen for glucuronide conjugation, decreasing the extent of conjugation of these other drugs.
Chemical Properties
Salicylamide is a odorless white or slightly pink crystals. It is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable solutions can be formed at pH 9 through ionization of the phenolic group.
Uses
salicylamide is an analgesic, fungicide, and anti-inflammatory ingredient used to soothe the skin. It is an aromatic amide.
Uses
Salicylamide is used in combination with both aspirin and caffeine in the over-the-counter pain remedies. It is used as an analgesic and as an antipyretic.
Definition
ChEBI: The simplest member of the class of salicylamides derived from salicylic acid.
General Description
Salicylamide, o-hydroxybenzamide, is a derivative of salicylicacid that is fairly stable to heat, light, and moisture. Itreportedly exerts a moderately quicker and deeper analgesiceffect than aspirin because of quicker CNS penetration. Its metabolismdiffers from aspirin, because it is not metabolized tosalicylic acid but rather excreted exclusively as the ether glucuronideor sulfate. Thus, as a result of lack of contributionfrom salicylic acid, it has a lower analgesic and antipyretic efficacythan that of aspirin. However, it can be used in place ofsalicylates for patients with a demonstrated sensitivity to salicylates.It is also excreted much more rapidly than other salicylates,which accounts for its lower toxicity. It is available inseveral nonprescription products, in combination with acetaminophenand phenyltoloxamine (e.g., Rid-A Pain compound,Cetazone T, Dolorex, Ed-Flex, Lobac) or with aspirin,acetaminophen, and caffeine (e.g., Saleto, BC Powder).
Air & Water Reactions
Salicylamide darkens on exposure to air. . Insoluble in water.
Reactivity Profile
Salicylamide is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Salicylamide may be sensitive to prolonged exposure to light.
Hazard
One of the primary side effects associated with Salicylamide is gastrointestinal discomfort. Allergic reactions are another potential side effect. It can also have effects on the liver. Prolonged use or high doses may lead to liver damage, which can present as jaundice (yellowing of the skin or eyes), dark urine, or pale stools.
Fire Hazard
Flash point data for Salicylamide are not available; however, it is probably combustible.
Clinical Use
Whereas salicylamide is reported to be as effective as aspirin as an analgetic/antipyretic and is effective in relieving pain associated with arthritic conditions, it does not appear to possess useful anti-inflammatory activity. Thus, indications for the treatment of arthritic disease states are unwarranted, and its use is restricted to the relief of minor aches and pain at a dosage of 325 to 650 mg three or four times per day. Its effects in humans are not reliable, however, and its use is not widely recommended.
Synthesis
A method for the preparation of salicylamide, in a 1000 liter stainless steel reactor, adding 150 kg of methyl salicylate, 420 kg of toluene, heating to 40 ~ 45 , continuous ventilation of ammonia for ammonia reaction, the reaction temperature is controlled at 40 ~ 45 , the reaction pressure is controlled at 0.25 ~ 0.35 MPa, the ammonia reaction stops the ventilation of ammonia after 5 hours, and then heating to recover toluene and reaction Then toluene and methanol, a by-product of the reaction, were heated and recovered, and the remaining material was cooled down to 20 , crystallized and centrifuged to obtain salicylamide with a yield of 97.8%.
Purification Methods
Crystallise the amide from water or repeatedly from CHCl3 [Nishiya et al. J Am Chem Soc 108 3880 1986]. [Beilstein 10 IV 169.] The anilide [87-17-2] M 213.2, m 135o crystallises from H2O. [Beilstein 12 H 500, 12 I 268, 12 II 256, 12 944.]
Salicylamide Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8615350571055 | Sibel@chuanghaibio.com | China | 8753 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5242 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18126 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 973 | 58 |
| airuikechemical co., ltd. | +86-18353166132 | sales02@airuikechemical.com | China | 983 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 | admin@hblongbang.com | China | 972 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +86-15350851019; +8615350851019 | admin@86-ss.com | China | 999 | 58 |
Related articles
- Salicylamide: Melanin-Inducing Activity, Antiviral Derivatives & Antigonorrheal Potential
- Salicylamide induces melanin via Mitf/tyrosinase, its derivatives have broad antiviral activity, and it combats multidrug-resi....
- Nov 4,2025
- What is the function and Uses of Salicylamide?
- Salicylamide is an over-the-counter drug popularly used as an analgesic and antipyretic.
- Nov 2,2024
View Lastest Price from Salicylamide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2026-02-18 | Salicylamide
65-45-2
|
0.99 | RongNa Biotechnology Co.,Ltd | ||||
 |
2026-02-13 | Salicylamide
65-45-2
|
US $0.00 / Kg/Drum | 1KG | 98%-102%; USP | 10 TONS | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2026-02-11 | Salicylamide
65-45-2
|
US $99.00-80.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD |
-

- Salicylamide
65-45-2
- 0.99
- RongNa Biotechnology Co.,Ltd
-

- Salicylamide
65-45-2
- US $0.00 / Kg/Drum
- 98%-102%; USP
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Salicylamide
65-45-2
- US $99.00-80.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
65-45-2(Salicylamide)Related Search:
1of4





