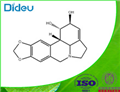
LYCORINE
- CAS No.
- 476-28-8
- Chemical Name:
- LYCORINE
- Synonyms
- LYCORIN;LYCORINE;Licorine;Amaryline;Belamarine;amarylline;narcissine;NSC 401360;NSC 683873;LYCORINE HCL
- CBNumber:
- CB6226644
- Molecular Formula:
- C16H17NO4
- Molecular Weight:
- 287.31
- MDL Number:
- MFCD00221746
- MOL File:
- 476-28-8.mol
- MSDS File:
- SDS
| Melting point | 253-255℃ (dec.) |
|---|---|
| alpha | D16 -129° (c = 0.16 in 98% alc) |
| Boiling point | 429.61°C (rough estimate) |
| Density | 1.53 |
| refractive index | 1.5500 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | powder |
| pka | 13.55±0.40(Predicted) |
| color | White to off-white |
| optical activity | -12916 (c 0.16, 98% ethanol) |
| InChI | InChI=1S/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15?,16+/m0/s1 |
| InChIKey | XGVJWXAYKUHDOO-KDMZPGMDSA-N |
| SMILES | [C@H]1(O)[C@]2([H])C3N(CCC3=C[C@@H]1O)CC1C2=CC2OCOC=2C=1 |
| FDA UNII | I9Q105R5BU |
LYCORINE price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | L0445 | Lycorine | 476-28-8 | 250MG | $311 | 2025-07-31 | Buy |
| Biorbyt Ltd | orb105513 | Lycorine >98%,Standard References Grade | 476-28-8 | 20mg | $569.5 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FL65705 | (-)-Lycorine | 476-28-8 | 500mg | $300 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PXT0000542 | LYCORINE 95.00% | 476-28-8 | 100G | $3577.61 | 2021-12-16 | Buy |
| ChemScene | CS-0008781 | Lycorine >98.0% | 476-28-8 | 50mg | $80 | 2021-12-16 | Buy |
LYCORINE Chemical Properties,Uses,Production
Description
Lycoris radiate, a traditional Chinese medicine (TCM), is the bulb of the amaryllidaceous Lycoris radiata herb. It has been applied for clinical purposes for centuries.
It is firstly recorded in Tujing Bencao and mainly used for the pyogenic infections.
According to A Supplement to Compendium of Materia Medica, lycoris radiate may
be used for treating acute throat trouble, phlegm node, baihuodan, and pulmonary
abscess.
There are about 20 Lycoris species in the world, which are widely distributed in
China and Japan. Lycoris radiate is an amazing horticultural plant with a graceful
shape and a bright color. In TCM, it is acrid in taste and neutral in nature, with functions of detoxication, easy expectoration, and diuresis and emesis promotion.
According to the modern medicine, lycoris radiate is considered to be in favor of the
central nervous system and cardiovascular system.
The main active ingredients extracted from the lycoris herbs are about 40 alkaloids with various contents. Pharmacological tests indicate that galanthamine, lycorine, lycoramine, lycorenine, and crinine are the major effective medicinal
ingredients. Lycorine may be used to treat amebic dysentery and against cancer.
Moreover, galanthamine, dihydrogalanthamine, and lycoramine may be used to treat infantile paralysis and restore nerve functions and against traumatic paraplegia, etc. Lycoris radiate is famous because galanthamine has been approved by FDA
as an anti-Alzheimer disease drug. Lycoris radiate is the only natural source for
galanthamine with extremely low content (<0.02%).
Physical properties
Appearance: colorless prismatic crystal. Solubility: insoluble in water; sparingly soluble in ethyl alcohol and diethyl ether. Melting point: 275–280?°C (decomposition). Specific optical rotation: right-handed optical rotation with a specific optical rotation of ?129° (98% ethyl alcohol).
History
In 1895, Morishima successfully extracted lycorine from the bulb of Lycoris radiata.
However, its structure was unidentified until in 1935. In 1959, its stereochemical
structure was dissected by monocrystal.
The solvent extraction method, chromatographic separation, and resin absorption are commonly used for lycorine extraction. However, lycorine obtained from
these techniques is not pure enough and often mixed with other alkaloids. The great
differences in the efficacies of different alkaloids prevent such blending from being
directly used. Furthermore, the separation and purification process so required has
an adverse effect on and limits the development and utilization of the medicinal
value of lycorine.
Uses
Lycorine is an analgesic, more so than aspirin, and a hypotensive, as are caranine and galanthine . The analgesic activity exhibited by the Amaryllidaceae alkaloids is attributed to their resemblance to the morphine and codeine skeletons. Lycorine also has antiarrhythmic action, and lycorine hydrochloride is a strong broncholytic. In fact, lycorine shows a relaxant effect on an isolated epinephrine-precontracted pulmonary artery and increases contractility and the rate of an isolated perfused heart. These effects are mediated by stimulation of b-adrenergic receptors.
Lycorine also has a strong inhibitory effect on parasite (Encephalitozoon intestinalis) development and antifungal activity against Candida albicans. Additionally, lycorine has antifeedant, antimalarial, emetic, anti-inflammatory, antiplatelet , as well as antifertility activities. Galanthine, in turn, shows mild in vitro activity against Tripanosoma brucei rhodesiense and Plasmodium falciparum.
Definition
ChEBI: An indolizidine alkaloid that is 3,12-didehydrogalanthan substituted by hydroxy groups at positions and 2 and a methylenedioxy group across positions 9 and 10. Isolated from Crinum asiaticum, it has been shown to exhibit antimalarial activit .
Indications
Injection: 25?mg/ml, for resistance to amebic protozoa and treatment of intraintestinal/extraintestinal amebiasis. Subcutaneous injection: 25–50? mg/injection and 50?mg/day.
Pharmacology
Great progress has been made in exploration of the pharmacological activities and
mechanisms of lycorine and its derivatives in recent years.
1. Effect on Central Nervous System
Lycorine can accelerate the mice’s conditioned reflex of motor and defense
nature. A mouse intraperitoneally injected with lycorine at 2?mg/kg and a rabbit
intramuscularly injected with lycorine at 12 or 20?mg/kg are exposed to a good
sedative effect. For a mouse and a rat injected with lycorine at 12?mg/kg and
15? mg/kg, respectively, the sleep time of hexobarbital sodium, pentobarbital
sodium, and miltown is extended. According to the hot plate test, the analgesic
actions of morphine and rhizoma corydalis are better in a mouse intraperitoneally injected with lycorine at 12?mg/kg.
2. Effect on Cardiovascular System
Intravenous injection of lycorine results in a slight antihypertensive effect in
anesthetized dog, cat, and rabbit, while no inhibitory effect is observed in isolated heart of toad.
3. Anti-inflammatory Effect
The lycorine may stimulate the pituitary gland-adrenocortical function, which
is possibly relevant to its anti-inflammatory action. Intravenous or subcutaneous
injection of lycorine at 3? mg/kg significantly inhibits formaldehyde-induced
(rabbit) and albumen-induced (rat) foot swelling. Its inhibitory effect on albumeninduced foot swelling in rat was abolished when adrenal gland was removed.
4. Effect on Smooth Muscle
Lycorine can excite the isolated uteri of guinea pig and rabbit, which is free
from the counteraction by diphenhydramine. The isolated uterus of rat is excited
by a small dose of lycorin but inhibited by a large dose. This effect is relevant to
the inhibition by lycorine on cholinesterase.
5. Emetic Effect
Lycorine has a good emetic action. The incubation period for emesis is similar to that of ipecine and longer than that of apomorphine, with low toxicity
reported. Therefore, it is sometimes used as the emetic for food poisoning.
6. Antitumor Effect
According to the in?vivo experiment, lycorine can inhibit the anaerobic glycolysis of the ascites tumor cells of mice but has no effect on their respiration and
aerobic glycolysis. The in?vitro test indicates that, however, lycorine can lead to
a significant inhibition on the aerobic glycolysis of tumor cells but little effect on
their respiration and anaerobic glycolysis. Lycorine is capable of inhibiting adenosine triphosphatase, which is possibly relevant to its cytotoxicity.
7. Antiparasitic and anti-malaria effect
Dihydrolycorine is better than ipecine in terms of the counteraction against
amebic dysentery, with lower toxicity. Thus, it has the potentiality to be a better
drug against amebic dysentery. Not only that, dihydrolycorine can be used
against paragonimiasis as well.
8. Other Effects
Upon the subcutaneous injection of a small amount of lycorine, the blood
glucose is observed to be reduced slightly in the target rabbit or rat, with the
epinephrine-induced hyperglycemia in the rat relieved. A large amount, however,
can only result in a significant rise of blood. Lycorine, similar to SKF-525A, can
inhibit drug metabolism to a weak extent. The amebic protozoa can be killed by
lycorine. A rat intraperitoneally injected with lycorine at 6?mg/kg has more uric
acid excreted.
Clinical Use
Dihydrolycorine, generated through the hydrogenation of lycorine, has been used
clinically due to its better resistance against amebic dysentery and lower toxicity.
The amine salt made of lycorine has an antitumor effect in animals.
Lycorine exposure may cause skin irritation (red and swollen) and itching.
Nosebleed may be induced in case of inhalation. In case of overdose, it may cause
salivation, emesis, diarrhea, bradycardia, cold hands/feet, or even death due to
respiratory center paralysis. The major studies of clinical application are focused on
(1) antitumor effect, (2) effect on the central nervous system, (3) effect on the cardiovascular system, (4) anti-inflammatory effect, (5) effect on smooth muscle, and
(6) emetic effect.
Purification Methods
It crystallises as orange crystals from MeOH (m 281-283o), CHCl3/EtOH (m 272-274o), pyridine or from EtOH (m 277o, dec). It has been distilled under high vacuum. The hydrochloride has m 288o (from MeOH/HCl), and the picrate has m 196-197o(from EtOH), [Cook et al. J Chem Soc 4176 1954, Martin & Tu J Org Chem 46 3763 1981, Beilstein 27 II 547, 27 III/IV 6463.]
LYCORINE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253; +8618283602253 | jancyzheng@gcgreenpure.com | China | 954 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29852 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 | 52513593@qq.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49975 | 58 |
| SIMAGCHEM CORP | +86-5922680277 +86-13806087780 | sale@simagchem.com | China | 17346 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 | sales@chemnorm.com | CHINA | 2935 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354; +17819995354 | marketing@targetmol.com | United States | 32432 | 58 |
View Lastest Price from LYCORINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-10-03 | Lycorine
476-28-8
|
US $30.00-68.00 / mg | 98.71% | 10g | TargetMol Chemicals Inc. | ||
 |
2025-09-29 | LYCORINE; Lycoris radiata extract
476-28-8
|
US $0.00 / KG | 1KG | ≥98% HPLC | 1000KG | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2021-06-18 | LYCORINE USP/EP/BP
476-28-8
|
US $1.10 / g | 1g | 99.9% | 100 Tons Min | Dideu Industries Group Limited |
-

- Lycorine
476-28-8
- US $30.00-68.00 / mg
- 98.71%
- TargetMol Chemicals Inc.
-

- LYCORINE; Lycoris radiata extract
476-28-8
- US $0.00 / KG
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- LYCORINE USP/EP/BP
476-28-8
- US $1.10 / g
- 99.9%
- Dideu Industries Group Limited







