FMOC-4-AMINOBENZOIC ACID
- CAS No.
- 185116-43-2
- Chemical Name:
- FMOC-4-AMINOBENZOIC ACID
- Synonyms
- FMOC-PABA-OH;FMOC-P-ABZ-OH;FMOC-4-ABZ-OH;RARECHEM EM WB 0013;FMOC-4-AMINOBENZOIC ACID;Fmoc-PABA-OH Novabiochem;4-(FMOC-AMINO)BENZOIC ACID;N-FMOC-4-AMINOBENZOIC ACID;Fmoc-4-aminobenzoic acid98+%;4-(FMOC-AMINO)BENZOIC ACID 96+%
- CBNumber:
- CB9437320
- Molecular Formula:
- C22H17NO4
- Molecular Weight:
- 359.37
- MDL Number:
- MFCD00144888
- MOL File:
- 185116-43-2.mol
- MSDS File:
- SDS
| Melting point | ~277 °C (dec.) |
|---|---|
| Boiling point | 544.9±33.0 °C(Predicted) |
| Density | 1.352±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| pka | 4.29±0.10(Predicted) |
| form | powder |
| Appearance | White to off-white Solid |
| BRN | 7722986 |
| CAS DataBase Reference | 185116-43-2(CAS DataBase Reference) |
| UNSPSC Code | 12352209 |
| NACRES | NA.26 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H335-H319-H315 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 10 | |||||||||
| HS Code | 2924 29 70 | |||||||||
| HazardClass | IRRITANT | |||||||||
| NFPA 704 |
|
FMOC-4-AMINOBENZOIC ACID price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 8.52219 | Fmoc-PABA-OH Novabiochem? | 185116-43-2 | 1g | $28.6 | 2025-07-31 | Buy |
| Sigma-Aldrich | 8.52219 | Fmoc-PABA-OH Novabiochem? | 185116-43-2 | 5G | $71.8 | 2025-07-31 | Buy |
| Sigma-Aldrich | 8.52219 | Fmoc-PABA-OH Novabiochem? | 185116-43-2 | 25g | $295 | 2025-07-31 | Buy |
| Sigma-Aldrich | 47307 | Fmoc-4-Abz-OH ≥96.0% (TLC) | 185116-43-2 | 1g | $83.1 | 2025-07-31 | Buy |
| TRC | F603565 | Fmoc-4-aminobenzoicacid | 185116-43-2 | 500mg | $65 | 2021-12-16 | Buy |
FMOC-4-AMINOBENZOIC ACID Chemical Properties,Uses,Production
Chemical Properties
White powder
Uses
Fmoc-4-aminobenzoic acid is used in the improved rapid synthesis of oligo(benzamide) block copolymers.
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
Synthesis
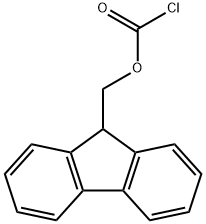
28920-43-6
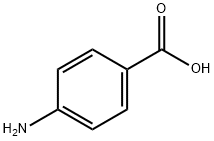
150-13-0

185116-43-2
Under an inert atmosphere, p-aminobenzoic acid (10 g, 73 mmol) was dissolved in anhydrous N-methylpyrrolidone (NMP, 50 mL). Subsequently, a solution of anhydrous NMP (50 mL) of 9-fluorenylmethyl chloroformate (Fmoc-Cl, 18.9 g, 73 mmol) was slowly added dropwise. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the mixture was slowly poured into 400 mL of water to precipitate the product. The colorless precipitate was collected by filtration and washed with plenty of water. Finally, the product was dried under vacuum at 120 °C to afford N-Fmoc-p-aminobenzoic acid (24.8 g, 94% yield). The melting point of the product was 215 °C (decomposition). Nuclear magnetic resonance hydrogen spectrum (1H-NMR, 300 MHz, DMSO-d6): δ 4.33 (t, J = 6.25 Hz, 1H), 4.54 (d, J = 6.25 Hz, 2H), 7.33-7.45 (m, 4H), 7.57 (d, J = 7.35 Hz, 2H), 7.76 (d, J = 7.35 Hz, 2H), and 7.89 (m, 4H), 10.08 (s, 1H), 12.69 (s, 1H). NMR carbon spectrum (13C-NMR and DEPT, 300 MHz, DMSO-d6): δ 46.61 (CH2), 65.83 (CH), 117.5 (CH), 120.22 (CH), 124.48 (C), 125.14 (CH), 127.17 (CH), 127.74 (CH), 130.48 ( CH), 140.86 (C), 143.31 (C), 143.74 (C), 153.31 (C), 167.03 (C). Infrared spectra (IR, ν, cm-1): 3344, 2970, 2887, 2660, 2544, 1709, 1673, 1610, 1592, 1526, 1511, 1411, 1311, 1282, 1221, 1052, 850, 736. reversed-phase high performance liquid chromatography (RP-HPLC) retention time: 26.6 Minutes. Mass spectrometry (FD-MS): m/z (%) = 359.1 (100), 360.1 (17.4); calculated value [C22H17NO4] = 359.1.
References
[1] Tetrahedron Letters, 2013, vol. 54, # 8, p. 753 - 756
[2] Chemical Communications, 2013, vol. 49, # 40, p. 4555 - 4557
[3] Journal of Chemical Research, Miniprint, 1998, # 10, p. 2736 - 2753
[4] Chemical Communications, 2014, vol. 50, # 14, p. 1691 - 1693
FMOC-4-AMINOBENZOIC ACID Preparation Products And Raw materials
Raw materials
Preparation Products
FMOC-4-AMINOBENZOIC ACID Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| GenicBio Limited | +86-21-37621270 | service@genicbio.com | CHINA | 991 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19552 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49730 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30230 | 58 |
| GL Biochem (Shanghai) Ltd. | +86-021-61263370 +86-18901917034 | CHINA | 3904 | 58 | |
| GL Biochem(Binhai) Ltd. | +86-021-61263370 +8615301650015 | wuqiuyan@glschina.com | China | 2975 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 21769 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | info@dycnchem.com | China | 52846 | 58 |
185116-43-2(FMOC-4-AMINOBENZOIC ACID)Related Search:
1of4






