| Structure |
Chemical Name |
CAS |
MF |
 |
Pharmaceutical film coating premix (stomach soluble) (medicinal excipients) |
|
|
 |
Medicinal white vaseline |
|
|
 |
tape adhesive for skin medicine |
|
|
 |
Lithium carbonate (medicinal) |
|
|
 |
Medicinal dipotassium hydrogen phosphate |
|
|
 |
DryobalanopsaromaticaGwaertn.f. |
|
|
 |
Potassium dihydrogen phosphate (medicinal excipients) |
|
|
 |
ベロニカオフィシナリス花/葉/茎エキス |
85117-19-7 |
|
 |
Saccharin sodium (pharmaceutical excipient) |
|
|
 |
Borax (pharmaceutical excipient) |
|
|
 |
Taurine (pharmaceutical excipient) |
|
|
 |
Medicinal iron powder |
|
|
 |
Australian pharmaceutical grade tea tree oil |
|
|
 |
Distearylphosphatidylcholine (medicinal excipient) |
|
|
 |
Refined corn oil (medicinal excipients) |
|
|
 |
Succinic acid (oral) (medicinal excipients) |
|
|
 |
Dipalmitoylphosphatidylcholine (for injection) (medicinal excipients) |
|
|
 |
Methoxy polyethylene glycol-poly(D,L-lactic acid) (for injection) (medicinal excipients) |
|
|
 |
Starch pharmaceutical grade |
|
|
 |
Potato starch (medicinal excipients) |
|
|
 |
Sucrose stearate (medicinal excipients) |
|
|
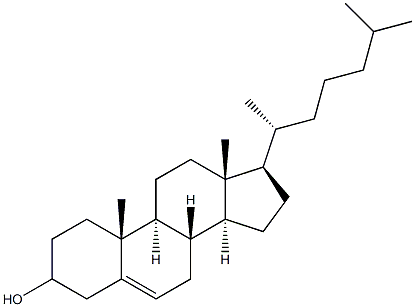 |
Cholesterol (for injection) (medicinal excipients) |
|
C27H46O |
 |
Triethanolamine (medicinal excipients) |
|
|
 |
Methyl cellulose (medicinal excipients) |
|
|
 |
Soybean phospholipid (medicinal excipient) |
|
|
 |
Emulsifier 400 do |
|
|
 |
Hydroxyethyl Cellulose |
|
|
 |
Pharmaceutical ammonium acetate |
|
|
 |
Medicinal boric acid |
|
|
 |
Pharmaceutical grade sodium carboxymethyl starch |
|
|
 |
Microcrystalline cellulose M102CG (medicinal excipients) |
|
|
 |
Methoxy polyethylene glycol-polylactic acid block copolymer (medicinal excipient) |
|
|
 |
Film coating premix (medicinal excipients) |
|
|
 |
Sugar coating premix (medicinal excipients) |
|
|
 |
Hydrochloric acid (medicinal excipients) |
|
|
 |
Film coated premixed adjunct (enteric soluble) (medicinal excipients) |
|
|
 |
West yellow gum (medicinal excipients) |
|
|
 |
Egg yolk lecithin (for injection) (medicinal excipients) |
|
|
 |
Polyethylene glycol monomethyl etherified polylactic acid (medicinal excipients) |
|
|
 |
Triacetin (triacetate glyceride) (medicinal excipients) |
|
|
 |
Starch Pharm |
|
|
 |
Medicinal corn oil |
|
|
 |
マンドレイク根エキス |
|
|
 |
Medicinal potassium acetate |
|
|
 |
High purity medicinal solid sodium methoxide |
|
|
 |
Pharmaceutical grade potassium chloride |
|
|
 |
Poloxamer 407 (pharmaceutical excipient) |
|
|
 |
humid medicinal materials |
|
|
 |
salicylic acid inclusion complex |
|
|
 |
Vitamin E (DL-Α-tocopherol) (medicinal excipients) |
|
|
 |
Egg yolk lecithin (medicinal excipients) |
|
|
 |
Urea (medicinal excipients) |
|
|
 |
Ammonium sulfate (medicinal excipients) |
|
|
 |
Calcium diphosphate (medicinal excipients) |
|
|
 |
Potassium sorbate (medicinal excipients) |
|
|
 |
Carbon dioxide (medicinal excipients) |
|
|
 |
Benzoic acid (medicinal excipients) |
|
|
 |
Medicinal alcohol |
|
|
 |
Paraffin wax (SASOLWAX 2697) (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 1000 |
|
|
 |
Glycerin (medicinal excipients) |
|
|
 |
Polyethylene glycol 400 (for injection) (medicinal excipients) |
|
|
 |
Sulfobutyl betacyclodextrin sodium (medicinal excipients) |
|
|
 |
Ethanol (99.5%) (medicinal excipients) |
|
C2H6O |
 |
PolyacrylicResin |
|
|
 |
Pharmaceutical grade polyethylene glycol 6000 |
|
|
 |
Oseltamivir Diastereomer I |
|
|
 |
CORN STARCH PHARMACEUTICAL GRADE |
|
|
 |
Cross-linked povidone (medicinal excipients) |
|
|
 |
Wheat starch (medicinal excipients) |
|
|
 |
Medicinal hypromellose hollow capsule |
|
|
 |
Pharmaceutical film coated premixed adjunct (enteric soluble) (medicinal excipients) |
|
|
 |
Polyacrylic resin latex (gastric collapse) (medicinal excipients) |
|
|
 |
Medicinal hydroxypropyl starch hollow capsule |
|
|
 |
サラシミツロウ |
8006-40-4 |
|
 |
Pharmaceutical grade polyacrylic resin No. 3 |
|
|
 |
Pharmaceutical film coating premixes |
|
|
 |
Pharmaceutical sodium sulfite (anhydrous) |
|
|
 |
Sodium thiosulfate (medicinal excipients) |
|
|
 |
Magnesium oxide (oral) (medicinal excipients) |
|
|
 |
Ethyl cellulose (medicinal excipients) |
|
|
 |
Ethyl cellulose aqueous dispersion (medicinal excipients) |
|
|
 |
Polyacrylic resin latex solution for coating premix (medicinal excipients) |
|
|
 |
Pharmaceutical grade povidone K90 |
|
|
 |
Tartaric acid pellet core (medicinal excipient) |
|
C4H6O6 |
 |
Medicinal enteric-soluble hypromellose hollow capsule |
|
|
 |
Carbomer homopolymer type A (medicinal excipients) |
|
|
 |
Medicinal hollow capsule |
|
|
 |
Lanolin (medicinal excipients) |
|
|
 |
GOMPHRENA OFFICINALIS ROOT EXTRACT |
|
|
 |
Soybean oil (for injection) (pharmaceutical excipient) |
|
|
 |
Medicinal silica gel dryer |
|
|
 |
Polyester bottle (medicinal) |
|
|
 |
Pharmaceutical grade poloxamer 188F68 |
|
|
 |
Rhubarb (medicinal rhubarb) |
|
|
 |
Carbomer homopolymer C type 980 NF (medicinal excipient) |
|
|
 |
Docusate sodium (medicinal excipients) |
|
|
 |
Xanthan gum (medicinal excipients) |
|
|
 |
Yamanite oleate (Span 80) (medicinal excipients) |
|
|
 |
Laurel Yamanashi (Span 20) (medicinal excipients) |
|
|