| Structure |
Chemical Name |
CAS |
MF |
 |
Pharmaceutical grade sodium carboxymethyl cellulose |
|
|
 |
Total ionic strength regulator PH5.0-5.5 |
|
|
 |
Thymol (medicinal excipients) |
|
|
 |
Calcium chloride (medicinal excipients) |
|
|
 |
Hydroxyethyl ester (medicinal excipients) |
|
|
 |
Glycolide lactide copolymer (5050) (for injection) (medicinal excipients) |
|
|
 |
Benzalkonium chloride (medicinal excipients) |
|
|
 |
Ascorbyl palmitate (medicinal excipient) |
|
|
 |
PSZ104 |
|
|
 |
PRAMIPEXOLE DIMER IMPURITY II |
|
|
 |
Methacrylic acid-ethyl acrylate copolymer and its aqueous dispersion, partially neutralized copolymer (pharmaceutical excipient) |
|
|
 |
Medicinal enteric-coated gelatin hollow capsules |
|
|
 |
Enteric-coated film coating premix (pharmaceutical excipients) |
|
|
 |
Cassava starch (pharmaceutical excipient) |
|
|
 |
Hydroxypropyl cellulose (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 8000 |
|
|
 |
Polymethacrylic resin latex (medicinal excipients) |
|
|
 |
Glycerides, coco mono-, di- and tri-, hydrogenated |
91744-42-2 |
|
 |
Blank micropellets sucrose type, medicinal micropellets core |
|
|
 |
Medicinal pellets (starch type) (medicinal excipients) |
|
|
 |
Talc powder (medicinal excipients) |
|
|
 |
Yellow Vaseline (medicinal excipients) |
|
|
 |
Microcrystalline wax (medicinal excipients) |
|
|
 |
Octadecyl alcohol (medicinal excipients) |
|
|
 |
Methacrylic acid copolymer type C (medicinal excipients) |
|
|
 |
Water based castor oil |
|
|
 |
Pharmaceutical grade polyethylene glycol |
|
|
 |
Red iron oxide (medicinal excipients) |
|
|
 |
Medicinal ink (yellow) (medicinal excipients) |
|
|
 |
783 type medicinal activated carbon |
|
|
 |
Needle, and a pharmaceutically acceptable activated reagent |
|
|
 |
Tartaric acid (medicinal excipients) |
|
|
 |
Methanesulfonic acid (medicinal excipients) |
|
|
 |
DL-tartaric acid (medicinal excipients) |
|
|
 |
Anhydrous sodium acetate (medicinal excipients) |
|
|
 |
Succinic acid (medicinal excipients) |
|
|
 |
Activated carbon (for injection) (medicinal excipients) |
|
|
 |
Acetic acid-ammonium acetate buffer (pH4.8) |
|
|
 |
Trihydrate medicinal sodium acetate |
|
|
 |
Medicinal hydrochloric acid |
|
|
 |
Potassium chloride (medicinal excipients) |
|
|
 |
Disodium hydrogen phosphate (medicinal excipients) |
|
|
 |
Sodium dihydrogen phosphate (medicinal excipients) |
|
|
 |
Silica (SYLYSIA 770FCP) (medicinal excipients) |
|
|
 |
Polyacrylic resin III (medicinal excipients) |
|
|
 |
Glyceric acid (medicinal excipients) |
|
|
 |
Tricaprylin (medicinal excipients) |
|
|
 |
エブリコエキス |
|
|
![ビスオクタデカン酸(R)-1-[[[(2-アミノエトキシ)ヒドロキシホスフィニル]オキシ]メチル]エチレン](/CAS/GIF/1069-79-0.gif) |
ビスオクタデカン酸(R)-1-[[[(2-アミノエトキシ)ヒドロキシホスフィニル]オキシ]メチル]エチレン |
1069-79-0 |
C41H82NO8P |
 |
20-Epi-16’-decarbomethoxy 19,20-conoduramine |
|
C41H54N4O3 |
 |
クロスカルメルロースナトリウム |
74811-65-7 |
Null |
 |
Sodium octanoate (medicinal excipients) |
|
|
 |
Plant-derived recombinant human serum albumin (pharmaceutical excipient) |
|
|
 |
Carbomer homopolymer C type 980 NF (medicinal excipient) |
|
|
 |
Disodium edetate (medicinal excipients) |
|
|
 |
Sodium benzoate (medicinal excipients) |
|
|
 |
Methanol (medicinal excipients) |
|
|
 |
Disodium edetate (for injection) (medicinal excipients) |
|
C10H14N2Na2O8 |
 |
Pharmaceutical grade pregelatinized starch |
|
|
 |
Huang Dan for medicine |
|
|
 |
Powdered cellulose (medicinal excipients) |
|
|
 |
Sucrose core (medicinal excipients) |
|
|
 |
Anhydrous sodium sulfite (medicinal excipients) |
|
|
 |
beta-Alanine, N-coco alkyl derivs., sodium salts |
68608-68-4 |
unspecified |
 |
Barm pharm grade |
|
|
 |
Medicinal calcium lactate |
|
|
 |
99.7% glycerol (medicinal excipients) |
|
|
 |
Azelaic acid (medicinal excipients) |
|
|
 |
Light magnesium oxide (medicinal excipients) |
|
|
 |
Gelatin hollow capsule |
|
|
 |
Magnesium stearate (medicinal excipients) |
|
|
 |
Dimethicone (medicinal excipients) |
|
|
 |
Light liquid paraffin (medicinal excipients) |
|
|
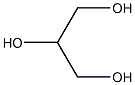 |
Glycerin (for injection) (medicinal excipients) |
|
C3H8O3 |
 |
Hexadecanol (medicinal excipient) |
|
|
 |
Polyvinyl alcohol (medicinal excipients) |
|
|
 |
合成ミツロウ |
71243-51-1 |
|
 |
Ferric chloride,medicinal |
|
FeCl3·6H20 |
 |
Dextrin (pharmaceutical excipients) |
|
|
 |
85% glycerin (pharmaceutical excipient) |
|
|
 |
Cetyl alcohol (medicinal excipients) |
|
|
 |
Sodium stearyl fumarate (medicinal excipients) |
|
|
 |
Yellow dextrin |
|
(C6H10O5)x |
 |
Anhydrous medicinal sodium acetate |
|
|
 |
Medicinal PVC hard tablets |
|
|
 |
Pharmaceutical film coating premix (porcine liver color) |
|
|
 |
Silica (SYLYSIA 770FCP) (medicinal excipients) |
|
|
 |
Pharmaceutical grade polyethylene glycol 8000 |
|
|
 |
Film coating premix (stomach soluble type) (medicinal excipients) |
|
|
 |
Protamine sulfate (pharmaceutical excipient) |
|
|
 |
Silicified microcrystalline cellulose (medicinal excipients) |
|
|
 |
Low-substituted hydroxypropyl cellulose (medicinal excipients) |
|
|
 |
Sodium bicarbonate (medicinal excipients) |
|
|
 |
Polyammonium methacrylate I (medicinal excipients) |
|
|
 |
Pregelatinized starch (medicinal excipients) |
|
|
 |
Paraffin (medicinal excipients) |
|
|
 |
Medicinal gelatin hollow capsule (ozone sterilization) |
|
|
 |
Yellow iron oxide (medicinal excipients) |
|
|
 |
Medicinal ink (red) (medicinal excipients) |
|
|
 |
ACETIC ACID-AMMONIUM ACETATE |
|
C4H11NO4 |