- CH5132799
-

- $58.00 / 1mg
-
2026-02-02
- CAS:1007207-67-1
- Min. Order:
- Purity: 99.41%
- Supply Ability: 10g
|
| | CH5132799 Basic information |
| Product Name: | CH5132799 | | Synonyms: | CH5132799;CH5132799,5-(7-(Methylsulfonyl)-2-Morpholino-6,7-dihydro-5H-pyrrolo[2,3-d]pyriMidin-4-yl)pyriMidin-2-aMine;[5-[7-Methylsulfonyl-2-(morpholin-4-yl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl]pyrimidin-2-yl]amine;5-(7-(Methylsulfonyl)-2-Morpholino-6,7-dihydro-5H-pyrrolo[2,3-d]pyriMidin-4-yl)pyriMidin-2-aMine;[5-[7-Methylsulfonyl-2-(morpholin-4-yl)-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl]pyrimidin-2-yl]amine CH5132799;CH5132799;CH 5132799;CH-5132799;5-(7-(Methylsulfonyl)-2-morpholino-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-ami;CS-541 | | CAS: | 1007207-67-1 | | MF: | C15H19N7O3S | | MW: | 377.42 | | EINECS: | | | Product Categories: | Inhibitors;PI3K/Akt/mTOR;JAK;STAT;Akt;mTOR;PI3K | | Mol File: | 1007207-67-1.mol | 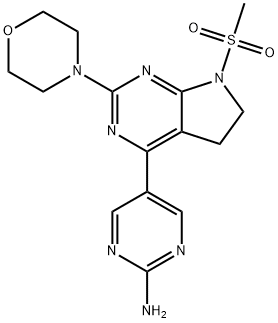 |
| | CH5132799 Chemical Properties |
| Boiling point | 751.1±70.0 °C(Predicted) | | density | 1.58 | | storage temp. | Store at -20°C | | solubility | Soluble in DMSO | | form | Powder | | pka | 3.96±0.20(Predicted) | | color | White to gray |
| | CH5132799 Usage And Synthesis |
| Uses | CH5132799 is a selective class I phosphoinositide 3-kinase (PI3K) inhibitor that targets human cancers harboring oncogenic PIK3CA mutations. CH5132799 also showed potent antiproliferative and antitumor activity. | | Biological Activity | ch5132799 is an inhibitor of class i phosphatidylinositol 3-kinase (pi3k) with ic50 value of 14nm against pi3kα [1].ch5132799 shows inhibitory effect on class i pi3k with ic50 values of 0.014μm, 0.12μm, 0.5μm and 0.036μm against pi3kα, pi3kβ, pi3kδ and pi3kγ, respectively. pi3kα is especially sensitive to ch5132799. ch5132799 is a selective inhibitor. it shows less effect on class ii pi3ks, class iii pi3k and mtor. for other 26 protein kinases, ch5132799 nearly has no inhibition with ic50 value of > 10μm. ch5132799 is found to have potent antitumor activity. it exerts ic50 values of 0.2μm, 0.032μm, 0.056μm and 0.12μm in hct116, kpl-4, t-47d and sk-ov-3 cell lines, respectively. moreover, ch5132799 is oral available in animal models. treatment of ch5132799 shows strong tumor regression at dose of 12.5 mg/kg in mice bearing human breast cancer xenografts [1]. | | Synthesis | GENERAL STEPS: To DMF (550 mL) under nitrogen protection were added 4-chloro-7-methanesulfonyl-2-morpholin-4-yl-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine (57.9 g, 181.6 mmol), 2-amino-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidine ( 48.2 g, 218.0 mmol), potassium phosphate (42.4 g, 199.8 mmol) and water (30 mL) and degassed. Subsequently, dichlorobis(triphenylphosphine)palladium(II) (1.3 g, 1.8 mmol) was added, degassed again, and then the reaction was stirred at 60 °C for 2 hours. After completion of the reaction, the reaction mixture was cooled in an ice bath, water (750 mL) was added and stirring was continued for 2 hours. The precipitate was collected by filtration and washed sequentially with water (240 mL) and acetone (240 mL) to give the crude product (75.6 g). The crude product (62.0 g) was suspended in water (1500 mL) and stirred at 50 °C for 1 hour. The suspension was filtered and washed sequentially with water (400 mL) and acetone (400 mL) to afford 5-(7-methylsulfonyl-2-morpholin-4-yl-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-amine as an off-white solid (53.7 g, 96% yield). | | in vivo | Mice bearing BT-474 tumors (n=14) are orally administered 50 mg/kg of Everolimus on a daily basis for 31 days and then randomized. After randomization, the mice are orally administered 50 mg/kg of Everolimus (n=4) and 12.5 mg/kg (n=5), and 25 mg/kg (n=5) of Izorlisib on a daily basis for 7 days. C, the vehicle-, Everolimus, and CH5132799-treated (25 mg/kg) tumors are resected at 4 hours after terminal administration in B, lysed, and analyzed by Western blotting. Izorlisib administration leads to a remarkable regression in a dose-dependent manner of the tumors regrown after the long-term Everolimus treatment. The tumors are resected at the end of treatment and analyzed by Western blotting with respect to PI3K pathway inhibition. Izorlisib suppresses various effectors in the PI3K pathway, including Akt, FoxO1, S6K, S6, and 4E-BP1, whereas Everolimus inhibits only phosphorylation of S6K and S6, both downstream effectors of mTORC1[1]. | | target | PI3Kα | | IC 50 | PI3Kα: 14 nM (IC50); PI3Kα-H1047R: 5.6 nM (IC50); PI3Kα-E545K: 6.7 nM (IC50); PI3Kα-E542K: 6.7 nM (IC50); PI3Kγ: 36 nM (IC50); PI3Kβ: 120 nM (IC50); PI3Kδ: 500 nM (IC50); PI3KC2β: 5.3 μM (IC50); mTOR: 1.6 μM (IC50) | | references | [1] ohwada j, ebiike h, kawada h, et al. discovery and biological activity of a novel class i pi3k inhibitor, ch5132799. bioorganic & medicinal chemistry letters, 2011, 21(6): 1767-1772. |
| | CH5132799 Preparation Products And Raw materials |
|