- Tanespimycin
-
- $59.00 / 10mg
-
2026-02-02
- CAS:75747-14-7
- Min. Order:
- Purity: 99.56%
- Supply Ability: 10g
- 17-AAG
-

- $0.00 / 1KG
-
2026-01-27
- CAS:75747-14-7
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 20TONS
- 17-AAG
-

- $1.00 / 1KG
-
2020-01-03
- CAS:75747-14-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200kg
Related articles - What is 17-AAG?
- 17-AAG is an Hsp90 inhibitor. This compound is a synthetic analog of geldanamycin, an antibiotic first purified in 1970 from S....
- Oct 20,2023
|
| | 17-AAG Chemical Properties |
| Melting point | 201-203°C | | Boiling point | 797.8±60.0 °C(Predicted) | | density | 1.21 | | RTECS | LX8932000 | | Fp | >110°(230°F) | | storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C | | solubility | DMSO: soluble | | pka | 8.62±0.70(Predicted) | | form | solid | | color | Purple or dark red | | Water Solubility | Soluble in DMSO at 150 mg/mL; soluble in ethanol at 5 mg/mL. Very poorly soluble in water | | Sensitive | Light Sensitive | | Stability: | Stable for 1 year from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | | Major Application | food and beverages
microbiology
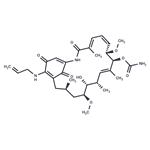
pathogen testing | | InChIKey | AYUNIORJHRXIBJ-HTLBVUBBSA-N | | SMILES | C12C(=O)C(=CC(=O)C=1NCC=C)NC(=O)C(C)=CC=C[C@H](OC)[C@@H](OC(=O)N)C(C)=C[C@H](C)[C@@H](O)[C@@H](OC)C[C@H](C)C2 |t:17,19,30| | | CAS DataBase Reference | 75747-14-7 |
| | 17-AAG Usage And Synthesis |
| Description |
Tanespimycin (17-AAG, CP127374, NSC-330507, KOS 953) (75747-14-7) is a potent HSP90 inhibitor with IC50 of 5 nM in a cell-free assay, having a 100-fold higher binding affinity for HSP90 derived from tumour cells than HSP90 from normal cells. Tanespimycin (17-AAG) induces apoptosis, necrosis, autophagy and mitophagy. Phase 3.
| | Targets | HSP90 5 nM. | | In vitro | 17-AAG, an analog of geldanamycin, exhibits greater than 100 times higher binding affinity for Hsp90 derived from HER-2-overexpressing cancer cells (BT474, N87, SKOV3 and SKBR3) or BT474 breast carcinoma cells with IC50 values of 5-6 nM. 17-AAG causes the degradation of HER2, HER3, Akt, and both mutant and wild-type androgen receptor (AR), leading to the RB-dependent G1 growth arrest of prostate cancer cells such as LNCaP, LAPC-4, DU-145, and PC-3 with IC50 values of 25-45 nM. [2] In addition to inducing apoptosis of Ba/F3 cells transformed with wild-type BCR-ABL with an IC50 of 5.2 μM, 17-AAG has the ability to induce apoptosis of cells transformed with imatinib mesylate-resistant T315I and E255K BCR-ABL mutants with IC50 values of 2.3 μM and 1.0 μM, respectively, by inducing the degradation of both wild-type BCR-ABL protein and mutants. | | Description | 17-AAG (75747-14-7) is a semi-synthetic analog of geldanamycin (Cat.# 10-1084) which is less toxic and more stable. 17-AAG selectively binds to and inhibits HSP90 from tumor cells. Anti-angiogenic activity. Cell permeable. | | Chemical Properties | Dark Purple Solid | | Uses | Potent inhibitor of heat shock protein 90 (Hsp90). 17-AAG is a less toxic analog than Geldanamycin. It induces apoptosis and displays antitumor effects. 17-AAG inhibits the activity of oncogenic proteins such as N-ras, Ki-ras, c-Akt, and p185erB2. | | Uses | Geldanamycin is a potent inhibitor of Hsp90 that exhibits severe hepatotoxicity when used in vivo. 17-AAG is an analog of geldanamycin which has potent in vivo activity and reduced toxicity. Like other Hsp90 inhibitors, 17-AAG has diverse anti-tumor actions and has potential in treating certain types of cancer. 17-AAG inhibits the growth of prostate cancer cell lines (IC50 = 25-45 nM). 17-AAG promotes the degradation of HER2 and induces growth arrest and apoptosis in breast cancer cells overexpressing HER2 (IC50 = 4-72 nM). | | Uses | 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) inhibits heat shock protein 90 (Hsp90), the expression of heat shock factor-1, and the activity of oncogenic proteins such as N-ras, Ki-ras, c-Akt, and p185erB2. In addition to its anti-tumor effects, 17-AAG also induces apoptosis. | | Definition | ChEBI: A 19-membered macrocyle that is geldanamycin in which the methoxy substituent attached to the benzoquinone moiety has been replaced by an allylamino group. It is a potent inhibitor of heat shock protein 90 (Hsp90). A less toxic analogue than geldanamycin,
t induces apoptosis and displays antitumour effects. | | General Description | Chemical structure: benzenoid | | Biological Activity | Inhibitor of heat shock protein 90 (Hsp90) chaperone activity, and an analog of geldanamycin (9,13-Dihydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-2-azabicyclo[16.3.1]docosa-4,6,10,18,21-pentaene-3,20,22-trione, 9-carbamate ). Subsequently inhibits the activity of oncogenic proteins such as p185 erbB-2 (IC 50 = 31 nM), N-ras, Ki-ras and c-Akt. Antitumor in vivo . | | Biochem/physiol Actions | 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) is a benzoquinone and is an analog of geldanamycin. | | Synthesis | The reaction of (+)-geldanamycin (5.1 mg, 9.0 μmol) with allylamine (10.0 μL, 0.13 mmol) was dissolved in chloroform (1.5 mL) at room temperature and stirred. The progress of the reaction was monitored by thin-layer chromatography (TLC), and complete conversion of geldanamycin was confirmed after 18 hours. The reaction mixture was subsequently washed with saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. Purification was carried out by rapid column chromatography on silica gel, the eluent being a mixed petroleum ether/ethyl acetate solvent, to give the target product as a purple solid (5.3 mg, 99% yield). The product characterization data were as follows: IR (KBr, cm1) 3464, 3333, 2958, 2929, 2825, 1728, 1691, 1652, 1571, 1485, 1372, 1323, 1189, 1101, 1057; UV (95% ethanol, λmax/nm) 332 (ε=2.0×10); 1H NMR (CDCl, 500 MHz) δ 9.14 (s, 1H), 7.28 (s, 1H), 6.93 (bd, J=11.5 Hz, 1H), 6.56 (bdd, J=11.5, 11.0 Hz, 1H), 6.38 (bt, J=6.0 Hz, 1H), 5.94-5.81 (m, 3H), 5.30- 5.24 (m, 2H), 5.17 (s, 1H), 4.82 (bs, 2H), 4.29 (bd, J=10.0 Hz, 1H), 4.21 (bs, 1H), 4.18-4.08 (m, 2H), 3.55 (ddd, J=9.0, 6.5, 2.0 Hz, 1H), 3.43 (ddd, J=9.0, 3.0 , 3.0 Hz, 1H), 3.34 (s, 3H), 3.25 (s, 3H), 2.72 (dqd, J=9.5, 7.0, 2.0 Hz, 1H), 2.63 (d, J=14.0 Hz, 1H), 2.34 (dd, J=14.0, 11.0 Hz, 1H), 2.00 (bs, 3H), 1.78 (d, J= 1.0 Hz, 3H), 1.78-1.74 (m, 2H), 1.74-1.67 (m, 1H), 0.99-0.95 (m, 6H); 13C NMR (CDCl, 125 MHz) δ 183.8 (18-C), 180.9 (21-C), 168.4 (1-C), 156.0 (7-OCNH), 144.6 (17-C), 141.2 (20-C), 135.8 (5-C), 134.9 (2-C), 133.7 (9-C), 132.7 (8-C), 132.5 (3'-C), 126.9 (4-C), 126.5 (3-C), 118.5 (3'-C), 108.8 (19-C), 108.7 (16-C), 81.6 (7-C), 81.4 (12-C), 81.2 (6-C), 72.6 (11-C), 57.1 (6- or 12-OCH), 56.7 (6- or 12-OCH), 47.8 (1'-C), 35.0 (13-C), 34.3 (15-C), 32.3 (10-C ), 28.4 (14-C), 22.9 (14-CH), 12.8 (8-CH), 12.6 (2-CH), 12.3 (10-CH); HRMS (FAB) m/z 586.3120 [M+H] (calculated value of 586.3129 for CHNO). | | storage | -20°C | | Background | 17-AAG is a semi-synthetic derivative of geldanamycin, demonstrating greater stability than its parent compound. It binds specifically to heat shock protein HSP90 in a manner similar to geldanamycin, but with weaker binding. Through specific binding with HSP90, 17-AAG has been shown to decrease levels of many proteins including androgen receptor, HER2, and Akt, while increasing levels of HSP90 in prostate cancer cell lines. 17-AAG binds to a conserved pocket in the HSP90 family of chaperone proteins and this occupation causes the degradation of several signaling proteins. | | References | [1] T W SCHULTE L M N. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin.[J]. Cancer Chemotherapy and Pharmacology, 1998, 42 4: 273-279. DOI:10.1007/s002800050817
[2] ADEELA KAMAL. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors[J]. Nature, 2003, 425 6956: 407-410. DOI:10.1038/nature01913
[3] ADEELA KAMAL Francis J B Marcus F Boehm. Therapeutic and diagnostic implications of Hsp90 activation.[J]. Trends in molecular medicine, 2004, 10 6: 283-290. DOI:10.1016/j.molmed.2004.04.006 |
| | 17-AAG Preparation Products And Raw materials |
|