|
|
| | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione Basic information |
| Product Name: | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione | | Synonyms: | Triallyl-s-triazine-2,4,6(1H,3H,5H)-trione, Stabilized, 98% 500GR;1,3,5-TRIALLYL-1,3,5-TRIAZINE-2,4,6(1H,3;Triallyl Isocyanurate(TAIC);Peroxide crosslinking agent;1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione, stabilized with MEHQ;1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione 98%;Triallyl Isocyanurate (stabilized with BHT), 96.0%(GC);1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione(TAIC) | | CAS: | 1025-15-6 | | MF: | C12H15N3O3 | | MW: | 249.27 | | EINECS: | 213-834-7 | | Product Categories: | rubber additive;rubber auxiliary;Allyl Monomers;Building Blocks;Chemical Synthesis;Crosslinkers;Crosslinking Agents;Heterocyclic Building Blocks;Materials Science;Polymer Science;Reagents for Polymerization;Triazines;Allyl Monomers;Monomers;Polymer Science;1025-15-6 | | Mol File: | 1025-15-6.mol | 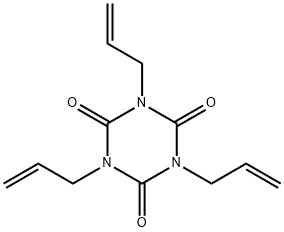 |
| | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione Chemical Properties |
| Melting point | 20.5°C | | Boiling point | 149-152 °C4 mm Hg(lit.) | | density | 1.159 g/mL at 25 °C(lit.) | | vapor pressure | 3.5 hPa (143 °C) | | refractive index | n20/D 1.513(lit.) | | Fp | >230 °F | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | 3.7g/l | | form | Liquid After Melting | | pka | -2.33±0.20(Predicted) | | color | Clear colorless to yellow | | Water Solubility | > 1 g/L (20 ºC) | | BRN | 225482 | | Stability: | May be prone to spontaneous polymerization. Commercial product is generally supllied with added stabilizer such as t-butylhydroquinone. Incompatible with peroxides, strong oxidizing agents, strong acids, strong bases. | | InChI | 1S/C12H15N3O3/c1-4-7-13-10(16)14(8-5-2)12(18)15(9-6-3)11(13)17/h4-6H,1-3,7-9H2 | | InChIKey | KOMNUTZXSVSERR-UHFFFAOYSA-N | | SMILES | C=CCN1C(=O)N(CC=C)C(=O)N(CC=C)C1=O | | LogP | 1.92 at 25℃ | | CAS DataBase Reference | 1025-15-6(CAS DataBase Reference) | | NIST Chemistry Reference | 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tri-2-propenyl-(1025-15-6) | | EPA Substance Registry System | Triallyl isocyanurate (1025-15-6) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36/37/39 | | WGK Germany | 1 | | RTECS | XZ1915000 | | F | 10 | | TSCA | TSCA listed | | HS Code | 2929109000 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Acute Tox. 4 Oral
STOT RE 2 | | Toxicity | LD50 orally in Rabbit: 700 mg/kg LD50 dermal Rat 2480 mg/kg |
| | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione Usage And Synthesis |
| Chemical Properties | colourless viscous liquid or white powder | | Uses | crosslinking accelerator agent for peroxide | | Uses | The Progress in Development of Dental Restorative Materials | | Synthesis Reference(s) | The Journal of Organic Chemistry, 59, p. 4931, 1994 DOI: 10.1021/jo00096a041 | | General Description | White crystalline solid. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Isocyanates and thioisocyanates, such as 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)]. | | Fire Hazard | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione is probably combustible. | | Flammability and Explosibility | Non flammable | | Synthesis | 6.45 g (50 mmol) of cyanuric acid, 670 mg (2.5 mmol) of triphenylphosphine (as an organophosphorus compound) and 25.8 g of xylene (as a solvent) were added to the reaction vessel. Under nitrogen protection, 266 mg of palladium-loaded activated carbon catalyst (trade name E101 NE/W, manufactured by Evonik Degussa Co., Ltd. which is a mixture of zero-valent metallic palladium and divalent palladium compounds with activated carbon in terms of palladium atoms) was added. The mixture was stirred at 95°C for 1 hour. Subsequently, 13.1 g (225 mmol) of propenol (as an allyl-type alcohol) was added slowly and dropwise over 1 h. The reaction lasted for 20 h, during which xylene, propenol, and generated water were removed by azeotropic distillation through a Dean-Stark apparatus. Upon completion of the reaction, the insoluble material was removed by filtration. Analysis of the filtrate showed 98.6% triallyl isocyanurate and 1.4% diallyl isocyanurate in the product based on the conversion of cyanuric acid, and no monoallyl isocyanurate generation was detected. | | References | [1] Patent: JP6288459, 2018, B2. Location in patent: Paragraph 0018; 0022; 0026-0029; 0031-0032; 0034-0035; 0037 |
| | 1,3,5-Tri-2-propenyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione Preparation Products And Raw materials |
| Raw materials | Cyanuric acid-->2,4,6-Triallyloxy-1,3,5-triazi-->Cyanuric acid trisodium salt-->1,3-Dichloropropene-->Sodium cyanate-->Allyl bromide-->allyl methanesulfonate-->Allyl alcohol-->Triphenylphosphine-->1,3-Cyclohexadiene, 5,5-dimethyl- | | Preparation Products | 1,3-bis(oxiranylmethyl)-5-(2-propenyl)-1,3,5-Triazine-2,4,6(1H,3H,5H)-trione-->Hexahydro-1,3,5-tris(2,3-dibromopropyl)-1,3,5-triazine-2,4,6-trione-->1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1-(2-oxiranylMethyl)-3,5-di-2-propen-1-yl- |
|