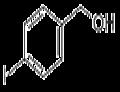
- 4-Iodobenzyl alcohol
-
- $1.00 / 1KG
-
2019-08-30
- CAS:18282-51-4
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1kg;10kg;100kg
|
| | 4-Iodobenzyl alcohol Basic information |
| | 4-Iodobenzyl alcohol Chemical Properties |
| Melting point | 72-75 °C (lit.) | | Boiling point | 136°C/5mmHg(lit.) | | density | 1.867±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | soluble in Methanol | | form | Crystalline Powder | | pka | 14.16±0.10(Predicted) | | color | White to pale yellow | | Sensitive | Light Sensitive | | BRN | 1931621 | | InChI | InChI=1S/C7H7IO/c8-7-3-1-6(5-9)2-4-7/h1-4,9H,5H2 | | InChIKey | CNQRHSZYVFYOIE-UHFFFAOYSA-N | | SMILES | C1(CO)=CC=C(I)C=C1 | | CAS DataBase Reference | 18282-51-4(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | DO8318020 | | HS Code | 29062990 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | 4-Iodobenzyl alcohol Usage And Synthesis |
| Chemical Properties | Solid | | Uses | 4-Iodobenzyl alcohol may be used in the preparation of:
- S-(4-Iodobenzyl) thioacetate
- S-(4-iodobenzyl) thiobenzoate
- fatty acid 4-iodobenzyl esters (FAIBEs)
- (4-trimethylsilylethynylphenyl)methanol
| | General Description | 4-Iodobenzyl alcohol is a benzyl alcohol derivative. | | Synthesis | Under the atmosphere of inert gas N2, 4-iodobenzoic acid (124.0 mg, 0.5 mmol) was added to the reaction flask after dehydration and deoxygenation treatment, pinacol borane (290 ??L, 2 mmol) was added by pipette gun, and the reaction was carried out at room temperature for 12 h. The reaction was removed from the glove box, and the reaction was analyzed by using homotrimethoxylbenzene (84.09 mg, 0.5 mmol) as the internal standard, and the sample was prepared by CDCl3 was dissolved, stirred for 10 min, sampled and prepared for NMR. The 1H yield was calculated to be 99%. NMR data of the product:1HNMR (400MHz, CDCl3): ??7.57(d,2H,ArCH),7.02(d,2H,ArCH),4.78(s,2H,OCH2),1.18(s,36H,CH3). To the sampling residue, 1 g of silica gel was added, and the borate ester was further hydrolyzed to alcohol by reacting at 50 ??C for 3 h with 3 mL of methanol as solvent. At the end of the reaction, the borate ester was extracted with ethyl acetate three times, and the organic layers were combined, dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure, and the product was purified by column chromatography on silica gel (100-200 mesh), and the ethyl acetate/hexanes (1:5) mixture was used as the eluent to obtain the pure Burkholderol was obtained in 94% isolated yield. |
| | 4-Iodobenzyl alcohol Preparation Products And Raw materials |
|