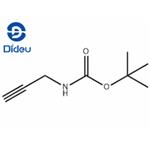
- N-Boc-propargylamine
-
- $0.00 / 25kg
-
2025-12-01
- CAS:92136-39-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS
- N-Boc-propargylamine
-

- $15.00 / 1KG
-
2021-07-13
- CAS:92136-39-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- N-Boc-propargylamine
-

- $15.00 / 1KG
-
2021-07-10
- CAS:92136-39-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | N-Boc-propargylamine Basic information |
| | N-Boc-propargylamine Chemical Properties |
| Melting point | 40-44 °C | | Boiling point | 170°C/14mmHg(lit.) | | density | 0.990±0.06 g/cm3(Predicted) | | Fp | 93°(199°F) | | storage temp. | 2-8°C | | solubility | Soluble in chloroform. | | form | Solid | | pka | 11.24±0.46(Predicted) | | color | Pale Yellow to Dark Yellow | | Sensitive | Moisture Sensitive | | InChI | InChI=1S/C8H13NO2/c1-5-6-9-7(10)11-8(2,3)4/h1H,6H2,2-4H3,(H,9,10) | | InChIKey | DSPYCWLYGXGJNJ-UHFFFAOYSA-N | | SMILES | C(OC(C)(C)C)(=O)NCC#C |
| Hazard Codes | Xn | | Risk Statements | 22-36/37/38-52/53 | | Safety Statements | 26-36/37-61 | | RIDADR | UN3259 | | WGK Germany | 3 | | HazardClass | 8 | | HS Code | 2924297099 | | Storage Class | 11 - Combustible Solids | | Hazard Classifications | Acute Tox. 4 Oral
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3 |
| | N-Boc-propargylamine Usage And Synthesis |
| Chemical Properties | Pale Yellow Low Melting Solid | | Uses | N-Boc-propargylamine is used in the preparation of triazolobenzylidene-thiazolopyrimidines which act as CDC25 phosphatase inhibitors. Also used in the synthesis of β-glucan polysaccharide analogs. | | Uses | N-Boc-propargylamine is used to prepare triazolobenzylidene-thiazolopyrimidines. which act as CDC25 phosphatase inhibitors. Further, it is used for the synthesis of beta-glucan polysaccharide analogs. In addition to this, it is involved in the Pauson-Khand (PK) reaction of norbornadiene and N-Boc-propargylamine to prepare 4,5-disubstituted cyclopentenones . | | Synthesis | Di-tert-butyl dicarbonate (17.5 g, 80.0 mmol, 1.0 eq.) was slowly added dropwise to a dichloromethane solution (160 mL) of propargylamine (5.49 mL, 80.0 mmol, 1.0 eq.) at 0 °C. The reaction mixture was stirred at 0 °C for 1 h before the solvent was removed by distillation under reduced pressure. The resulting colorless oil was dried overnight under high vacuum to afford N-Boc-aminopropargyl (12.4 g, quantitative yield) as a white solid, which can be used in subsequent reactions without further purification. The spectral data of the product were consistent with those reported in the literature: Rf = 0.38 (hexane/ethyl acetate, 9:1); melting point = 41-42 °C (literature value:[1] 40-44 °C).1H NMR (500 MHz, CDCl3): δ (ppm) 4.94 (broad peak, 1H), 3.91 (broad double peak, J = 2.5 Hz, 2H), 2.23 (triple peak, J = 2.5 Hz, 1H). 2.5Hz, 1H), 1.45 (single peak, 9H).13C NMR (126MHz, CDCl3): δ (ppm) 155.2, 80.1, 79.9, 71.1, 28.2. | | References | [1] Tetrahedron Letters, 2011, vol. 52, # 17, p. 2199 - 2202
[2] Organic Letters, 2014, vol. 16, # 9, p. 2430 - 2433
[3] European Journal of Organic Chemistry, 2015, vol. 2015, # 32, p. 7091 - 7113
[4] Patent: US2004/254231, 2004, A1
[5] Angewandte Chemie - International Edition, 2011, vol. 50, # 6, p. 1338 - 1341 |
| | N-Boc-propargylamine Preparation Products And Raw materials |
|