|
|
| | Ethyl N-(4-methylphenyl)sulfonylcarbamate Basic information |
| Product Name: | Ethyl N-(4-methylphenyl)sulfonylcarbamate | | Synonyms: | ethyl n-(4-methylphenyl)sulfonylcarbamate;Ethyl [(4-methylphenyl)sulphonyl]carbamate;N-(p-Tolylsulfonyl)carbamic acid ethyl ester;Tosylcarbamic acid ethyl ester;N-(4-methylphenyl)sulfonylcarbamic acid ethyl ester;3-ethyl-4-methylbenzenesulfonic acid carbamoyl ester;ethyl tosylcarbaMate;Gliclazide IMpurity C | | CAS: | 5577-13-9 | | MF: | C10H13NO4S | | MW: | 243.28 | | EINECS: | 226-952-9 | | Product Categories: | | | Mol File: | 5577-13-9.mol | 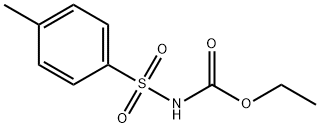 |
| | Ethyl N-(4-methylphenyl)sulfonylcarbamate Chemical Properties |
| Melting point | 82-84oC | | Boiling point | 272℃ at 100.8-101kPa | | density | 1.38 at 20℃ | | vapor pressure | 1.8-7.9Pa at 20-25℃ | | storage temp. | Sealed in dry,Room Temperature | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) | | form | Solid | | color | White | | LogP | 0.73-1.7 at 23℃ and pH2-7 | | Surface tension | 63.8mN/m at 1g/L and 20℃ |
| | Ethyl N-(4-methylphenyl)sulfonylcarbamate Usage And Synthesis |
| Uses | Tosylurethane (Gliclazide EP Impurity C) is an intermediate in the synthesis of Gliclazide (G409877), a sulfonylurea hypoglycemic agent. Used as an antidiabetic. | | Synthesis | GENERAL STEPS: Dissolve 4-methylphenylsulfonylcarbamate (Compound, CAS:31224-37-0) in selected alcohol (ethanol, 2 mL) in a microwaveable reaction vial. The reaction vial was sealed using an aluminum cap with a PTFE-faced septum. The reaction mixture was placed in a microwave reactor and radiantly heated at 100-120°C for 20-60 min. Upon completion of the reaction, the crude product was concentrated under reduced pressure and the residue was subsequently purified by silica gel column chromatography to finally obtain the target product, ethyl N-(4-methylphenyl)sulfonylcarbamate. | | References | [1] Tetrahedron Letters, 2016, vol. 57, # 13, p. 1476 - 1478 |
| | Ethyl N-(4-methylphenyl)sulfonylcarbamate Preparation Products And Raw materials |
|