- 1-Benzyl-3-Piperidinol
-

- $0.00 / 1kg
-
2024-06-11
- CAS:14813-01-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 tons
|
| | 1-Benzyl-3-piperidinol Basic information |
| Product Name: | 1-Benzyl-3-piperidinol | | Synonyms: | 1-Benzyl-3-hydroxypiperidine,99%;CIS-1-BENZYL-2-METHYL-3-AMINO PYRROLIDINE;1-N-BENZYL-3-HYDROXY-PIPERIDINE;N-BENZYL-3-HYDROXYPIPERIDINE;N-BENZYL-3-PIPERIDINOL;1-BENZYL-3-HYDROXYPIPERIDINE;1-BENZYL-PIPERIDIN-3-OL;1-BENZYL-3-PIPERIDINOL | | CAS: | 14813-01-5 | | MF: | C12H17NO | | MW: | 191.27 | | EINECS: | 238-881-0 | | Product Categories: | Piperidine;john's | | Mol File: | 14813-01-5.mol | 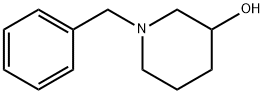 |
| | 1-Benzyl-3-piperidinol Chemical Properties |
| Boiling point | 140-142°C 6mm | | density | 1,056 g/cm3 | | refractive index | n20/D 1.549 | | Fp | >230 °F | | storage temp. | Inert atmosphere,Room Temperature | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 14.82±0.20(Predicted) | | form | clear liquid | | color | Colorless to Light yellow | | Sensitive | Hygroscopic | | BRN | 135964 | | InChI | InChI=1S/C12H17NO/c14-12-7-4-8-13(10-12)9-11-5-2-1-3-6-11/h1-3,5-6,12,14H,4,7-10H2 | | InChIKey | UTTCOAGPVHRUFO-UHFFFAOYSA-N | | SMILES | N1(CC2=CC=CC=C2)CCCC(O)C1 | | CAS DataBase Reference | 14813-01-5(CAS DataBase Reference) |
| Hazard Codes | T | | Risk Statements | 25-36/37/38 | | Safety Statements | 26-45 | | RIDADR | UN 2811 6.1/PG 3 | | WGK Germany | 2 | | HS Code | 29333990 |
| | 1-Benzyl-3-piperidinol Usage And Synthesis |
| Chemical Properties | Colorless liquid | | Uses | Reactant for bioresolution of tertiary amino ester protic ionic liquids using subtilisin
Reactant for synthesis of:
- Muscarinic M3 selective antagonists
- Rho kinase inhibitors
- Piperidine derivatives for investigations into α-adrenoreceptor direct activation
| | Synthesis | General procedure for the synthesis of 1-benzyl-3-piperidinol from 1-benzyl-3-piperidone: first, N-benzyl-3-piperidone hydrochloride was converted to a free base by the addition of an aqueous solution of K2CO3, which was subsequently extracted with ethyl acetate. Next, sodium borohydride (NaBH4) (0.438 g, 11.57 mmol) was slowly added to an ethanol solution containing N-benzyl-3-piperidone (2.19 g, 11.57 mmol) over 10 min. The reaction mixture was stirred overnight at room temperature and then concentrated under vacuum. The concentrated residue was dissolved in 1.0 N HCl and washed twice with diethyl ether. Afterwards, the aqueous phase was adjusted to pH 12 with 3.0N KOH and extracted three times with dichloromethane. The organic phases were combined and dried with Na2SO4, followed by vacuum concentration. The purity of the crude product was 100% (1.900 g, 86% yield) by IC/MS (EI) analysis: m/z 192.3 (M+1). | | Waste Disposal | 2,4-Dichloro-5-methoxypyrimidine is a halogen-containing aromatic ring organic compound, which is more harmful to the water environment. Therefore, undiluted or large amounts of 2,4-dichloro-5-methoxypyrimidine should not be exposed to groundwater, waterways, or sewage systems. | | References | [1] Patent: WO2005/26145, 2005, A2. Location in patent: Page/Page column 98
[2] Process Biochemistry, 2017, vol. 56, p. 90 - 97 |
| | 1-Benzyl-3-piperidinol Preparation Products And Raw materials |
|