|
|
| | 3''-ACETYL-2''-HYDROXY-4-(4-PHENYLBUTOXY)BENZANILIDE Basic information |
| Product Name: | 3''-ACETYL-2''-HYDROXY-4-(4-PHENYLBUTOXY)BENZANILIDE | | Synonyms: | N-(3-acetyl-2-hydroxyphenyl)-4-(4-phenylbutoxy)benzamide;3-[4-(4-Phenylbutoxy)benzoylamino]-2-hydroxyacetophenone;3-[4-(4-phenyl-1-butoxy)benzoyl]amino-2-hydroxyacetophenone;3'-[4-(4-Phenylbutoxy)benzoylamino]-2'-hydroxyacetophenone (Pranlukast);Benzamide, N-(3-acetyl-2-hydroxyphenyl)-4-(4-phenylbutoxy)-;N-(3-acetyl-2-hydroxyphe ny\-a-@- phenylbutoxy)benzamide 136450-06-1;3''-acetyl-2''-hydroxy-4-(4-phenylbutoxy)benzanilide;3’-[4-(4-Phenylbutoxy)benzoylamino]-2’-hydroxyacetophenone | | CAS: | 136450-06-1 | | MF: | C25H25NO4 | | MW: | 403.47 | | EINECS: | 416-150-9 | | Product Categories: | | | Mol File: | 136450-06-1.mol | 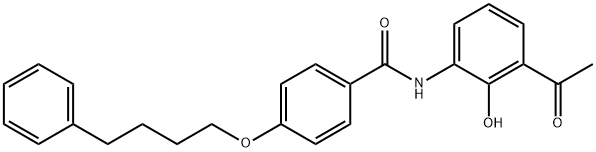 |
| | 3''-ACETYL-2''-HYDROXY-4-(4-PHENYLBUTOXY)BENZANILIDE Chemical Properties |
| Boiling point | 528.0±50.0 °C(Predicted) | | density | 1.213±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | pka | 9.50±0.40(Predicted) | | Appearance | Light yellow to yellow Powder | | InChI | InChI=1S/C25H25NO4/c1-18(27)22-11-7-12-23(24(22)28)26-25(29)20-13-15-21(16-14-20)30-17-6-5-10-19-8-3-2-4-9-19/h2-4,7-9,11-16,28H,5-6,10,17H2,1H3,(H,26,29) | | InChIKey | NTUBQTVFDLDHRH-UHFFFAOYSA-N | | SMILES | C(NC1=CC=CC(C(C)=O)=C1O)(=O)C1=CC=C(OCCCCC2=CC=CC=C2)C=C1 |
| Risk Statements | 53 | | Safety Statements | 61 |
| | 3''-ACETYL-2''-HYDROXY-4-(4-PHENYLBUTOXY)BENZANILIDE Usage And Synthesis |
| Synthesis | The general procedure for the synthesis of 3-[4-(4-phenylbutoxy)benzoylamino]-2-hydroxyacetophenone from 4-phenylbutoxybenzoic acid and 3-amino-2-hydroxyacetophenone was as follows: first, 270 g of 4-(phenylbutoxy)benzoic acid was mixed with 270 g of thionyl chloride, and the reaction was carried out for 3 hours at 50 °C. Upon completion of the reaction, the unreacted thionyl chloride was recovered by distillation under reduced pressure. Subsequently, the reaction product was dissolved in 270 g of dichloromethane under nitrogen protection and cooled to room temperature. Another 151 g of 3-amino-2-hydroxyacetophenone was dissolved in 200 g of dichloromethane, 160 g of pyridine was added, and then a dichloromethane solution of 4-(phenylbutoxy)benzoyl chloride prepared as described above was slowly added dropwise under the condition of an ice bath, and the reaction temperature was controlled to be no more than 10 ℃. After the dropwise addition was completed, the reaction was continued at 10°C for 2 hours. At the end of the reaction, the pH of the reaction mixture was adjusted to 2-3 with dilute hydrochloric acid, the organic layer was separated, washed to neutrality with dichloromethane, dried over anhydrous sodium sulfate, filtered and concentrated to give a reddish brown solid. Finally, it was recrystallized by a solvent mixture of petroleum ether and ethyl acetate (1:1, v/v) to give 334 g of the target product in 84% yield. | | References | [1] Patent: CN106588897, 2017, A. Location in patent: Paragraph 0019 |
| | 3''-ACETYL-2''-HYDROXY-4-(4-PHENYLBUTOXY)BENZANILIDE Preparation Products And Raw materials |
|