1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE manufacturers
|
| | 1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE Basic information |
| Product Name: | 1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE | | Synonyms: | 1-(2-PYRIMIDINYL)TETRAHYDRO-4(1H)-PYRIDINONE;1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE;1-Pyrimidin-2-yl-piperidin-4-one95%;1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE 95%;4-Oxo-1-(pyrimidin-2-yl)piperidine, 2-(4-Oxopiperidin-1-yl)pyrimidine;4-Oxo-1-(pyrimidin-2-yl)piperidine;1-(2-pyrimidinyl)-2-piperidinone;4-Piperidinone, 1-(2-pyrimidinyl)- | | CAS: | 116247-92-8 | | MF: | C9H11N3O | | MW: | 177.2 | | EINECS: | | | Product Categories: | API intermediates | | Mol File: | 116247-92-8.mol | 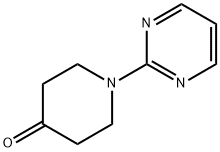 |
| | 1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE Chemical Properties |
| Melting point | 73-75°C | | Boiling point | 366.8±52.0 °C(Predicted) | | density | 1.225±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C, protect from light | | pka | 4.65±0.33(Predicted) | | Appearance | White to off-white Solid |
| Hazard Codes | Xi,Xn | | Risk Statements | 22 | | HazardClass | IRRITANT | | HS Code | 2933399990 |
| | 1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE Usage And Synthesis |
| Synthesis | The general procedure for the synthesis of 1-(pyrimidin-2-yl)piperidin-4-one from 2-chloropyrimidine and 4-oxopiperidone hydrochloride is as follows:
5.1 Preparation of 1-(pyrimidin-2-yl)piperidin-4-one:
A. A mixture of 4-piperidone monohydrate hydrochloride (4.84 g, 31.5 mmol), 2-chloropyrimidine (3.44 g, 30 mmol) and triethylamine (TEA, 10.04 mL, 72 mmol) was dissolved in ethanol (EtOH, 150 mL) and heated to reflux overnight. After completion of the reaction, the mixture was concentrated to near dryness and diluted with ethyl acetate (EtOAc, 400 mL). The EtOAc layer was washed sequentially with water (2 × 50 mL) and brine (2 × 50 mL). The aqueous layer was back-extracted with EtOAc (4 × 100 mL). All EtOAc layers were combined, dried with anhydrous sodium sulfate (Na2SO4), filtered and the solvent concentrated under reduced pressure. The residue was purified by ISCO fast chromatography (120 g silica gel column, elution procedure: hexane for 5 min, 0-80% EtOAc/hexane gradient for 70 min, and a final pure EtOAc elution for 15 min) to afford the target product 1-(pyrimidin-2-yl)piperidin-4-one (3.5 g, 66% yield).
The product characterization data are shown below:
HPLC analytical conditions: a Luna Phenyl-Hexyl 5 μm 4.6×50 mm column with 10 mM aqueous ammonium acetate as mobile phase A and acetonitrile as mobile phase B. Gradient elution (10-90% B, 3 min) was performed at a flow rate of 3 mL/min with retention times of 0.97 and 1.08 min.
Mass spectrum (MS): m/z 178 [M+H]+.
1H NMR (300 MHz, CDCl3): δ 2.52 (t, J = 6.29 Hz, 4H), 4.15 (t, J = 6.20 Hz, 4H), 6.60 (t, J = 4.67 Hz, 1H), 8.38 (d, J = 4.77 Hz, 2H). | | References | [1] Patent: WO2008/58064, 2008, A1. Location in patent: Page/Page column 17
[2] Patent: WO2008/58068, 2008, A1. Location in patent: Page/Page column 23
[3] Patent: WO2008/67120, 2008, A2. Location in patent: Page/Page column 23
[4] Patent: WO2008/67121, 2008, A2. Location in patent: Page/Page column 52
[5] Patent: US2006/258672, 2006, A1. Location in patent: Page/Page column 17 |
| | 1-PYRIMIDIN-2-YL-PIPERIDIN-4-ONE Preparation Products And Raw materials |
|