Product Code: E068001A
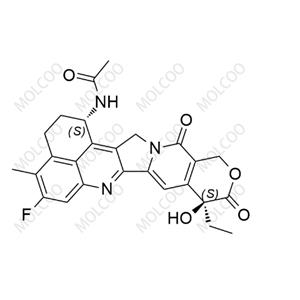
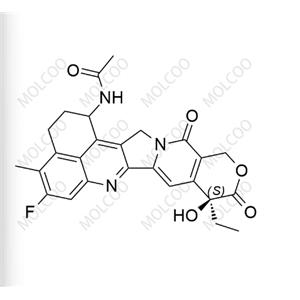
English Name: (1R,9S)-Exatecan (Mesylate)
English Alias: (1R,9S)-1-amino-9-ethyl-5-fluoro-9-hydroxy-4-methyl-2,3,12,15-tetrahydrobenzo[de]pyrano[3',4':6,7]indolizino[1,2-b]quinoline-10,13(1H,9H)-dione methanesulfonate
CAS No.:2231666-58-1
Molecular Formula: C₂₄H₂₂FN₃O₄·CH₄O₃S
Molecular Weight: 435.45 (free base) + 96.11 (methanesulfonate), total approx. 531.56
High Optical Purity: As the (1R,9S)-configured mesylate salt, it features a defined stereochemical structure, suitable for experimental scenarios requiring high-purity chiral compounds.
Enhanced Stability: The mesylate form improves the solubility and stability of the free base, facilitating storage and application.
Regulatory Compliance: Serves as a reference standard or impurity standard in drug development, meeting international regulatory requirements (e.g., ICH) for pharmaceutical impurity control.
Drug Development: Used in quality research of Exatecan-related drugs, including purity analysis, chiral isomer detection, and stability testing.
Analytical Reference: Acts as a standard for HPLC, NMR, and other analytical methods to establish or validate detection accuracy.
Pharmacological Research: Functions as a tool compound to study the activity and metabolic differences between this isomer and the parent Exatecan in vitro and in vivo.
Exatecan is a topoisomerase I inhibitor belonging to the camptothecin class of anticancer drugs. It exerts antitumor effects by inhibiting DNA topoisomerase I activity, preventing DNA replication and transcription. (1R,9S)-Exatecan (Mesylate), as the chiral isomer mesylate salt of Exatecan, requires strict quality control in drug development to ensure the safety and efficacy of the main drug. The introduction of mesylate salts is often used to improve physicochemical properties such as solubility and stability.
Synthesis Studies: Current synthesis methods for this compound primarily obtain the (1R,9S) configuration through chiral catalysis or resolution techniques, and mesylate salt preparation often involves salt-forming reactions to optimize crystallization conditions.
Activity Research: Some studies indicate that changes in chiral configuration may affect drug-target binding ability and toxicity, so comparative studies with the parent drug are necessary for evaluating its antitumor activity and safety.
Quality Control: In generic drug development, studies focus on analyzing the content of this impurity relative to the reference drug to assess its impact on formulation quality, with emphasis on analytical method development and stability investigation.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!











 China
China