Bempedoic Acid Impurity

WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information:
Product Number: B079023
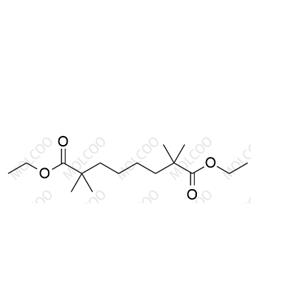
English Name: Bempedoic Acid Impurity 23
English Alias: diethyl 2,2,7,7-tetramethyloctanedioate
CAS Number: 77958-45-3
Molecular Formula: C₁₆H₃₀O₄
Molecular Weight: 286.41
Advantages:
High-purity standard:HPLC purity ≥99.0%, with structure confirmed by 1H NMR, 13C NMR, and HRMS, meeting the strict technical requirements of FDA and EMA for impurity reference standards, enabling precise qualitative and quantitative analysis.
Excellent stability:Stable for 36 months when stored at -20°C in the dark, with a degradation rate <1% after 30 days at room temperature in solution (e.g., methanol-acetonitrile system), suitable for long-term quality monitoring and stability studies of complex synthesis processes.
Strong process relevance:As a characteristic impurity from ester condensation reactions or chain-growth side reactions in bempedoic acid synthesis, it directly tracks process risks of excessive alkylation or insufficient catalyst selectivity during diester condensation.
Applications:
Pharmaceutical quality control:Used for LC-MS/MS detection of Impurity 23 in bempedoic acid APIs and formulations, controlling its content ≤0.1% in accordance with ICH Q3A standards to ensure compliance with impurity limits for lipid-regulating drugs.
Synthesis process optimization:In ester condensation reactions, monitoring impurity content (e.g., reducing catalyst dosage from 2mol% to 0.5mol% to decrease impurity from 1.2% to 0.1%) optimizes reaction parameters to inhibit chain-growth by-product formation.
Analytical method development:Serves as a diester impurity reference standard for establishing specific detection methods, such as ultra-performance liquid chromatography-refractive index detection (UPLC-RI), achieving accurate quantification of non-UV-absorbing impurities (limit of quantitation LOQ=0.1μg/mL).
Toxicological research support:Provides samples for evaluating the potential toxicity of diester impurities, facilitating in vitro cell permeability assays and in vivo excretion kinetics studies to meet regulatory requirements for in-depth impurity safety assessment.
Background Description:
During bempedoic acid synthesis, uncontrolled ester condensation conditions (e.g., high temperature, strong alkalinity) may lead to intermolecular alkylation of diesters, generating diethyl 2,2,7,7-tetramethyloctanedioate (Impurity 23). The impurity’s long-chain diester structure may affect drug lipophilicity and metabolic pathways, increasing potential accumulation toxicity risks. According to the ICH Q3B guideline, such process-related impurities must be strictly controlled through process optimization and testing to ensure drug safety and batch consistency.
Research Status:
Innovations in detection technology:UPLC-MS/MS with atmospheric pressure chemical ionization (APCI) source is used, employing a C18 column (1.7μm, 2.1×100mm) and n-hexane-isopropanol (gradient elution) as the mobile phase, achieving a detection limit (LOD) of 0.05ppm to solve the problem of trace detection of non-UV-absorbing impurities.
Formation mechanism analysis:This impurity mainly originates from bimolecular ester condensation reactions, with significantly increased formation at high catalyst (e.g., sodium ethoxide) concentrations or prolonged reaction times. Introducing sterically hindered catalysts (e.g., potassium tert-butoxide) and low-temperature (-5℃) reactions can reduce impurity formation by over 95%.
Safety evaluation:In vitro red blood cell hemolysis tests showed no obvious toxicity at concentrations ≤500μg/mL, but mild adipose tissue hyperplasia was observed in high-dose groups (300mg/kg) during a 90-day rat feeding test. Based on toxicological data, a recommended limit of ≤0.07% is proposed.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!









 China
China