
Product Details
| Product Name: Fasudil Nitroso Impurity 25 | CAS No.: 55557-00-1 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C₅H₁₀N₄O₂ |
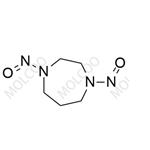
Fasudil Nitroso Impurity 25

WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Information
Product Number: F012025
English Name: Fasudil Nitroso Impurity 25
English Alias: 1,4-dinitroso-1,4-diazepane
CAS Number: 55557-00-1
Molecular Formula: C₅H₁₀N₄O₂
Molecular Weight: 158.16
Advantages
Well-defined and distinct structure: Contains a 1,4-diazepane skeleton with bis-nitroso (-NO) substituents, showing significant differences from the hexahydro-1H-1,4-diazepine core of Fasudil. It can be accurately identified by techniques such as HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The conjugated system of bis-nitroso groups and diazepane ring is stable under dark conditions. As a by-product of hexahydrodiazepine ring oxidation in Fasudil synthesis, it directly reflects the degree of amino nitrosation, improving the accuracy of process tracing;
High detection sensitivity: The conjugated system of nitroso groups has characteristic UV absorption (230-250nm), and combined with specific mass spectral response (m/z 159 [M+H]⁺), trace analysis can be achieved via LC-MS, compatible with detection systems for nitrogen heterocyclic drugs.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Fasudil Nitroso Impurity 25 in Fasudil APIs and formulations, ensuring residual nitroso impurities meet quality standards;
Synthesis process optimization: Optimizing amino protection/deprotection conditions of hexahydro-1H-1,4-diazepine ring by monitoring impurity content to reduce nitroso by-product formation;
Stability evaluation: Assessing the oxidative deterioration trend of Fasudil formulations during storage to support the development of appropriate storage conditions (e.g., light protection, low oxygen).
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS with optimized parameters based on characteristic fragment ions of bis-nitroso groups to achieve trace detection (detection limits up to ppb level);
Nitrosation mechanism analysis: Studying the formation kinetics of this impurity by simulating different pH and nitrite concentration conditions, clarifying the key influencing factors of amino bis-nitrosation in Fasudil;
Toxicological assessment: Evaluating the potential hazards of this bis-nitroso derivative through in vitro genotoxicity tests to provide a scientific basis for formulating strict impurity limit standards;
Inhibition strategy research: Developing nitrosation inhibitors (e.g., sulfite derivatives) or inert gas protection processes to inhibit the formation of this impurity during Fasudil production and storage
Product Information
Product Number: F012025
English Name: Fasudil Nitroso Impurity 25
English Alias: 1,4-dinitroso-1,4-diazepane
CAS Number: 55557-00-1
Molecular Formula: C₅H₁₀N₄O₂
Molecular Weight: 158.16
Advantages
Well-defined and distinct structure: Contains a 1,4-diazepane skeleton with bis-nitroso (-NO) substituents, showing significant differences from the hexahydro-1H-1,4-diazepine core of Fasudil. It can be accurately identified by techniques such as HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: The conjugated system of bis-nitroso groups and diazepane ring is stable under dark conditions. As a by-product of hexahydrodiazepine ring oxidation in Fasudil synthesis, it directly reflects the degree of amino nitrosation, improving the accuracy of process tracing;
High detection sensitivity: The conjugated system of nitroso groups has characteristic UV absorption (230-250nm), and combined with specific mass spectral response (m/z 159 [M+H]⁺), trace analysis can be achieved via LC-MS, compatible with detection systems for nitrogen heterocyclic drugs.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Fasudil Nitroso Impurity 25 in Fasudil APIs and formulations, ensuring residual nitroso impurities meet quality standards;
Synthesis process optimization: Optimizing amino protection/deprotection conditions of hexahydro-1H-1,4-diazepine ring by monitoring impurity content to reduce nitroso by-product formation;
Stability evaluation: Assessing the oxidative deterioration trend of Fasudil formulations during storage to support the development of appropriate storage conditions (e.g., light protection, low oxygen).
Background Description
Research Status
Detection method optimization: Using UPLC-MS/MS with optimized parameters based on characteristic fragment ions of bis-nitroso groups to achieve trace detection (detection limits up to ppb level);
Nitrosation mechanism analysis: Studying the formation kinetics of this impurity by simulating different pH and nitrite concentration conditions, clarifying the key influencing factors of amino bis-nitrosation in Fasudil;
Toxicological assessment: Evaluating the potential hazards of this bis-nitroso derivative through in vitro genotoxicity tests to provide a scientific basis for formulating strict impurity limit standards;
Inhibition strategy research: Developing nitrosation inhibitors (e.g., sulfite derivatives) or inert gas protection processes to inhibit the formation of this impurity during Fasudil production and storage
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $33.00/50mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-10-26 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-20 |








 China
China